

|
Mark Kirchhof, MD, PhD, FRCPC Head of Dermatology Division
|

|
Loukia Mitsos, MD, FRCPC Protoderma
|
The 30th European Academy of Dermatology and Venereology (EADV), held virtually from September 29th to October 2nd, 2021, showcased the latest innovations in dermatology and provided a platform for in-depth scientific exchange. On their 30th anniversary Congress, the EADV scientific programme showcased advances in science and clinical practice updates, which featured over 550 prominent speakers, ten plenary lectures, and 160 simulive sessions on cutting-edge topics, breaking news, free communication sessions, e-posters, and industry sessions included in the mix. At this year’s Congress, atopic dermatitis (AD) was covered in detail, from pathophysiology to the different treatment modalities.
The major theme in treatment was the ongoing introduction of new biologics and JAK inhibitors.1 Comparisons of baricitinib, upadacitinib, and abrocitinib versus dupilumab suggest a future where a range of individualized therapy options abound.2 Beyond the physical effects of AD, several presentations explored the psychosocial aspects, recognizing the need for greater physician understanding of the day-to-day struggles of children with AD and their families.3 Here are some highlights from this year’s programme, paving the way to a better quality of life for patients with AD and their loved ones.
ATOPIC DERMATITIS IS A SYSTEMIC DISEASE
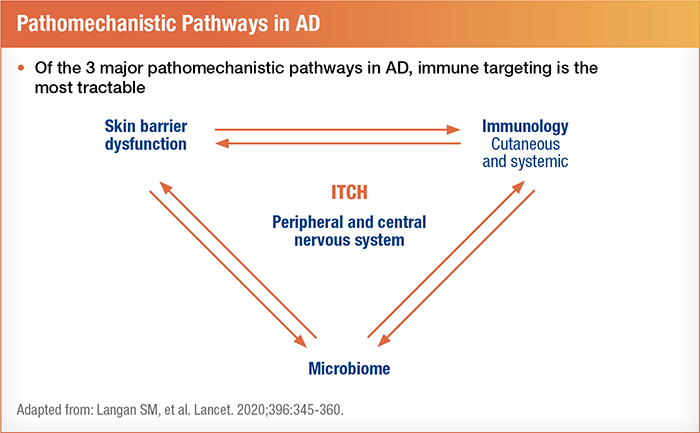
Some researchers have united the inside-out and outside-in theories of atopic dermatitis by incorporating environmental triggers, skin barrier defects, gut microbiome characteristics, and internal immune characteristics into a more unified pathogenesis. Viewpoints on AD as a chronic inflammatory condition are starting to mirror how we classify and think about psoriasis.4 Interestingly, compared to controls and patients with psoriasis, AD patients displayed higher immune activity in peripheral blood, including greater elevations in circulatory cytokines and activated T cells.4
Targeting inflammatory pathways identified as therapeutic options in other autoimmune conditions has proven successful in the treatment of AD. For example, a phase 2 study on etrasimod (an oral selective sphingosine 1-phosphate receptor modulator) significantly reduced AD symptoms for over 100 participants after 12 weeks.6 Etrasimod has already established a good safety profile and efficacy in the treatment of a range of conditions from Crohn's disease to alopecia areata.6
Addressing atopic dermatitis on an immunological level has proven a successful approach, and several more targeted treatments have shown promise. Phase 2 trials of monoclonal antibodies targeting the OX40-OX40L axis, which may be an early step in the AD inflammatory pathways, seem to achieve long-lasting disease control.1
Amlitelimab (KY1005) is a non-depleting anti-OX40L antibody ligand IgG4 monoclonal antibody that targets upstream mechanisms of inflammation as a once-monthly monotherapy.7 Researchers set up 19 recruitment sites throughout Germany, Poland, the UK, and Spain to evaluate its efficacy and safety profile. The low-dose amlitelimab group received a 200 mg loading dose and 100 mg maintenance doses every four weeks. The high-dose group received a 500 mg loading dose with 250 mg maintenance doses every four weeks. Data was compared against a placebo control group.
In the trial, treatment was given for the first 12 weeks, and the effects were observed at 16 weeks (59 participants) with a safety follow-up at 36 weeks (48 participants). Itch significantly reduced in both amlitelimab groups compared to placebo, and additional improvements in AD skin manifestations were noted.7 Although further investigation is needed, this early research supports the concept behind targeting OX40L with drugs like amlitelimab for people with AD.
Another phase 2 study explored this mechanism using KHK4083 (AMG 451). This IgG1 monoclonal antibody halts the clonal expansion of T-cells and blocks memory T-cell formation, inhibiting activated T-cells, including Th2.1 Patients were given either 150 mg of subcutaneous KHK4083 every four weeks, 300 mg every two weeks, 600 mg every two weeks, or 600 mg every four weeks for the entire 36-week study duration.1 A total of 273 study participants were randomized to receive a placebo for 18 weeks and switched to 600 mg of subcutaneous KHK4083 (every two weeks) during weeks 18 to 36. Results showed significant improvements in AD signs and symptoms at week 16 for those given KHK4083. In addition, positive effects associated with KHK4083 were sustained for 20 weeks after treatment was discontinued, with good tolerance and no significant safety concerns.1
Researchers have also been studying an oral molecule called RPT193, which inhibits the C-C motif chemokine receptor 4 (CCR4).8 In AD, Th2 cells accumulate in the skin and selectively express CCR4, which is associated with more severe AD symptoms, including itch. During a phase 1b trial, RPT193 was found to significantly improve AD symptoms compared to placebo within 29 days, providing benefits that continued for two weeks after treatment was stopped.8
A small study on spesolimab also looked at the effects on barrier dysfunction for AD patients. Spesolimab is a first-in-class humanized anti-interleukin 36 monoclonal IgG receptor antibody that showed clinically meaningful (but not yet statistically significant) improvements.9 A cohort of 51 male and female adult participants was randomized to receive four injections of spesolimab (600 mg) or placebo once every four weeks. Participants who reached an EASI75 score were considered "responders" and remained under observation for an additional 12 weeks without further treatment. Non-responders continued with spesolimab treatment for an extra 16 weeks. The improvements seen in responders continued through week 28 despite discontinuing treatment.9 No significant adverse side effects were observed. But unfortunately, this study had a very small sample size of just five participants.10
FURTHER TREATMENTS TARGETING AD USING MONOCLONAL ANTIBODIES
Several other monoclonal antibodies are under development, including an investigational drug called bermekimab, an IL-1α inhibitor. IL-1 is believed to accelerate inflammation from IL-13, IL-22, and IL-36.4 Almost all cells have a receptor for IL-1α, which is primarily secreted by keratinocytes.4 Previous research shows that a genetic IL-1 inhibitor deficiency results in severe immune-mediated skin inflammation. In addition, higher activation of IL-1 has been observed in the skin cells of adults and children with AD. Early results in a phase 2 open-label trial using 400 mg of bermekimab suggest that it produces a quick response in AD.4 However, at this time, no placebo-controlled studies have validated these results.
More robust data is emerging on tralokinumab and dupilumab. Research suggests that patients who respond well to tralokinumab (an IL-13 inhibitor) after 16 weeks sustain benefits at week 52 when given maintenance treatment every two weeks or every four weeks.11 Looking further out, an analysis of the ECZTEND trial checked in at the 2-year mark of an ongoing 5-year study on tralokinumab.12 Over two years, 345 participants received 300 mg injections of tralokinumab every two weeks with the option to incorporate topical corticosteroids after the initial 600 mg loading phase. Some participants entered the trial after having already been on tralokinumab consistently through parent trials. Others who were not on the drug upon entry showed levels of symptom control equivalent to other participants within 12 weeks of initiating treatment. Results indicated long-term AD improvements, including better sleep and reduced itch.12
Studies on dupilumab have demonstrated improvements in the skin barrier defects and systemic inflammatory markers, solidifying theories that AD manifestations may be reversed by targeting the Th2 axis and IL-4 and IL-13 cytokines.4 Significant benefits were documented within two weeks of beginning dupilumab treatment. Furthermore, positive effects achieved by week 16 were maintained throughout a 52-week observation period.5
An open-label extension trial on 300 mg dupilumab showed patient outcomes after 172 weeks of treatment.13 This interim analysis studied 253 adults weekly (sometimes in combination with topical therapies) from various phase 1 to 3 parent studies.14 PP-NRS (Peak Pruritus Numerical Rating Scale) scores started around 5 and improved rapidly within the first 16 weeks of treatment, ultimately settling at a mean value of 2.2 by the end of 172 weeks.13 Compared to baseline values, 98.7% of participants saw at least a 50% EASI score boost by week 172. EASI scores improved by 75% in 94.6% of participants and 90% in 82.6% participants.14 Additionally, no new safety concerns were identified with longer-duration treatment.13
DEVELOPMENTS IN SMALL MOLECULE JAK INHIBITORS
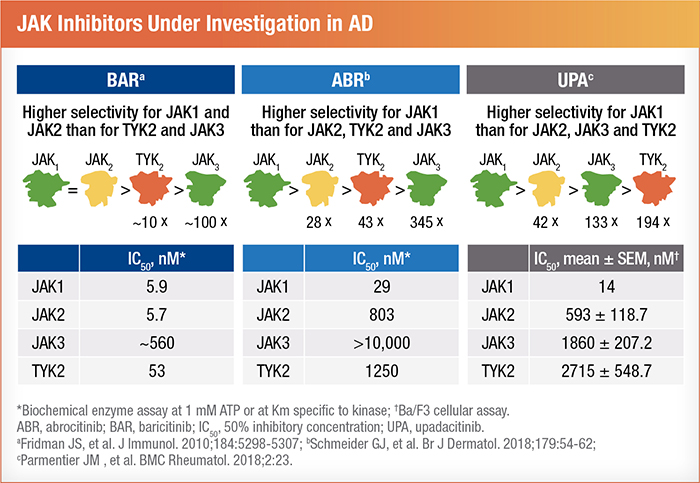
The expanded use of dupilumab has opened the door for comparison studies introducing newer JAK inhibitors. Currently, three JAK inhibitors have moved into advanced trial stages.5 Baricitinib received approval from the EMA and UK in the 4th quarter of 2020. Abrocitinib is approved for patients age 12 and older in the UK and is under review in the US.15 In Canada, upadacitinib is the only approved JAK inhibitor, but it remains under review in Europe and the US.5 Although all three of these drugs have the same target, they differ in how selective they are for individual pathways.5 For instance, while baricitinib is equally selective for JAK 1 and JAK 2, abrocitinib and upadacitinib predominately target JAK 1.5 It's hopeful that these different variations in specificity will enable more individualized and effective treatment of AD.4
The results of the phase 3 JADE COMPARE trial on abrocitinib were discussed.15
This graphic provides an overview of the study design:
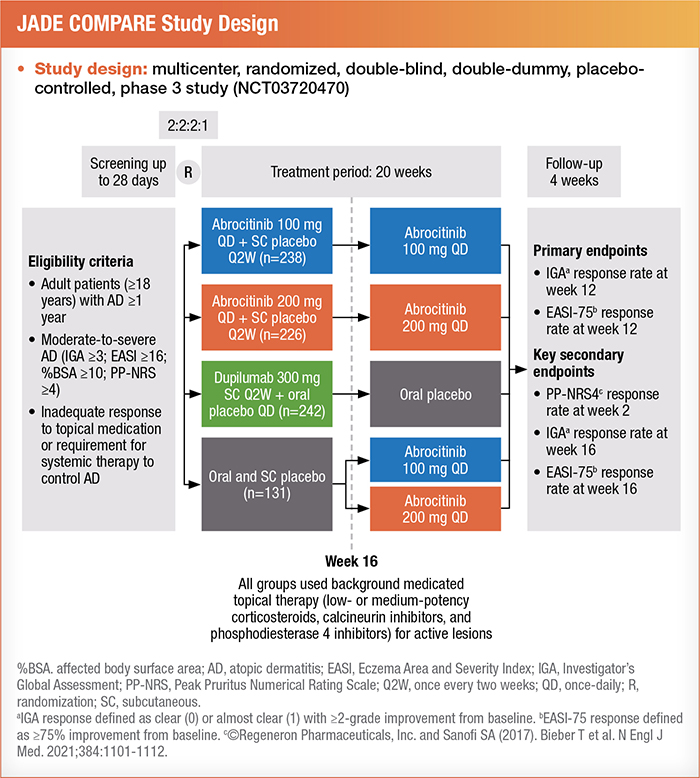
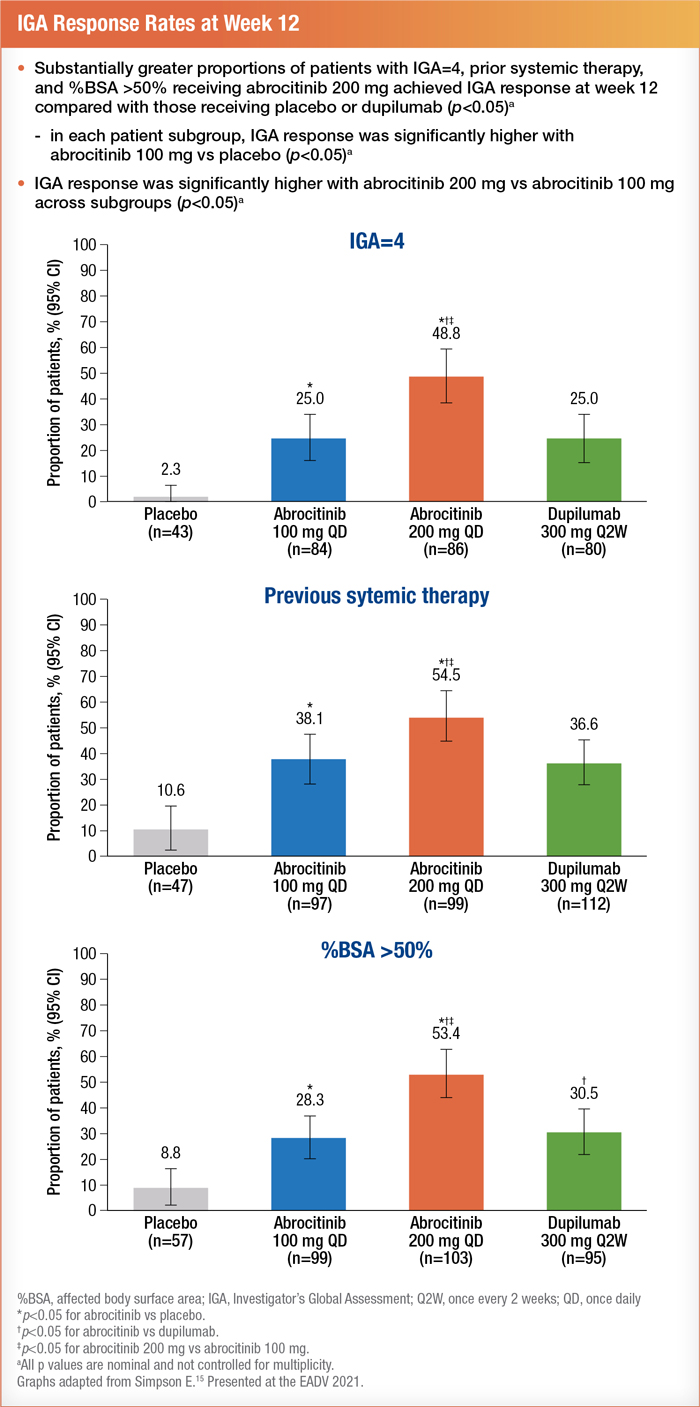
Patients with severe disease were divided into three subgroups. These included patients with an IGA of 4, a %BSA > 50, or a history of systemic immunosuppressant use (including systemic corticosteroids) for AD.15
Researchers analyzed data from 577 participants. Treatment effectiveness was measured at 12 weeks by an IGA score of 0 (clear) or 1 (almost clear) with an improvement of 2 or greater and an EASI response of 75% or more improvement.15 Results showed that 200 mg of abrocitinib was the most effective intervention for achieving positive results in each subgroup. Patients on a daily oral dose of 100 mg of abrocitinib achieved similar results to those on 300 mg of dupilumab (administered subcutaneously every two weeks after a 600-mg loading dose).16
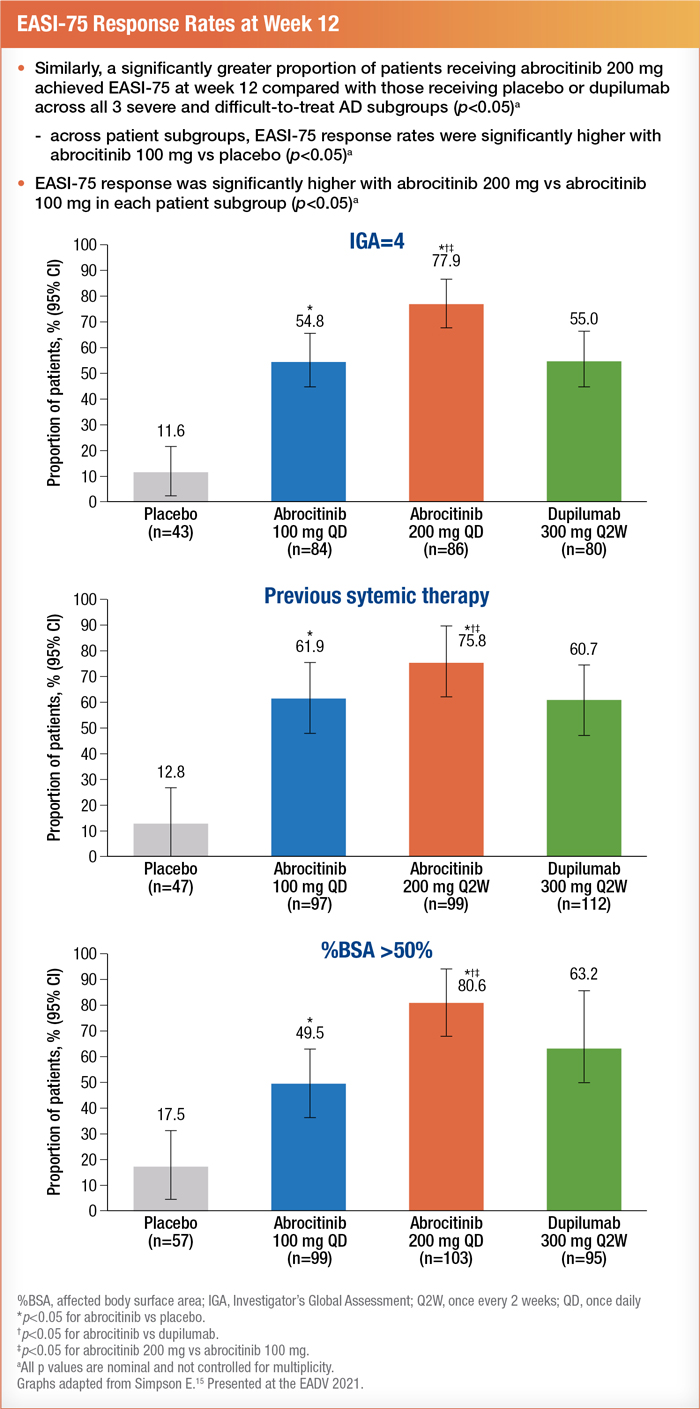
Patients on abrocitinib showed good tolerance with no new safety concerns.15 Researchers measured safety as all-cause treatment-emergent adverse events (TEAEs) and serious TEAEs. Of the 577 participants, 58.6% reported all-cause TEAEs, and 2.1% reported serious TEAEs. The most common side effects of abrocitinib were nasopharyngitis, nausea, acne, and headache.15
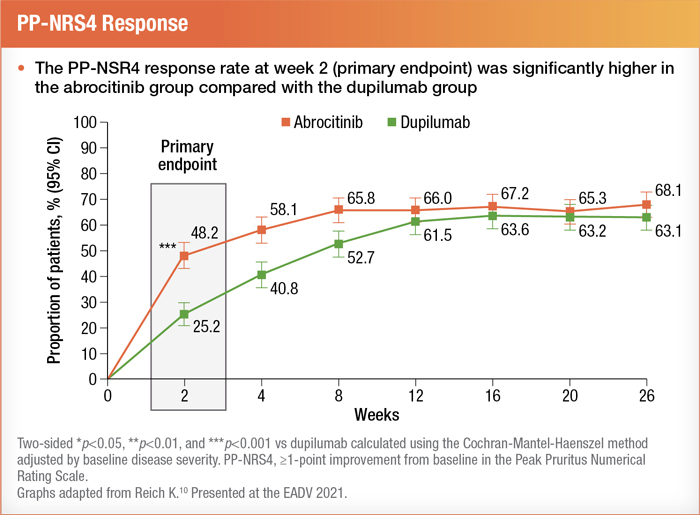
Abrocitinib was also trialed against dupilumab in a large head-to-head phase 3 study (JADE DARE) with over 700 participants. Results showed a rapid response for itch within just two weeks of initiating abrocitinib treatment.10 See below:
Furthermore, EASI-90 scores were significantly higher after four weeks of abrocitinib and maintained through 16 weeks.10
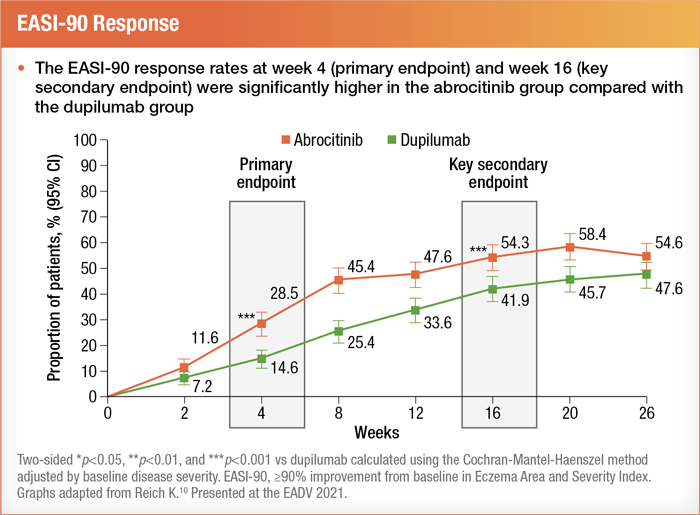
During the study, some trial participants continued the background use of topical therapies. Nonetheless, in his presentation, Dr. Kristian Reich noted that there were "more steroid-free EASI-90 days with abrocitinib compared with dupilumab."10 Additionally, for teens with AD, the phase 3 JADE TEEN study provided a glimpse of preliminary data suggesting that abrocitinib does not affect the immune response of adolescents receiving the Tdap vaccine.17
Switching patients who already take dupilumab over to upadacitinib was assessed in an open-label extension trial comparing the two molecules.18 After the initial six months, all participants continued on upadacitinib for six additional months. Upadacitinib was found particularly beneficial for those who didn't respond to dupilumab, with 40% of this second group achieving EASI100 once they switched to upadacitinib.18 The improvements started within four weeks of switching and were sustained through the end of the trial.
Upadacitinib provided a significant reduction in itch and other AD parameters.18 When comparing four body regions, researchers concluded that a higher percentage of upadacitinib patients achieved EASI75 than those on dupilumab in every area. These superior results started as soon as week 1 and continued through week 16, as shown below19:
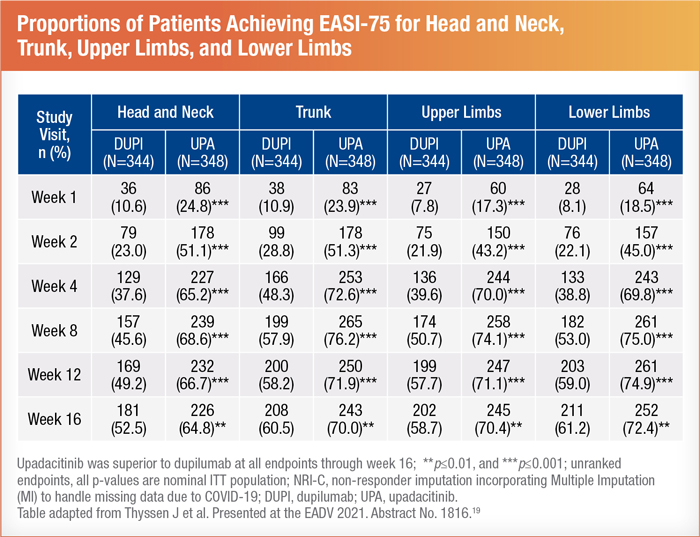
Participants were given a two-week washout period between treatments to minimize potential residual effects of dupilumab. As a result, the steep improvement curve is believed to have been caused by upadacitinib alone, but researchers acknowledged that there may have been some mixed effects.18 No new safety risks for upadacitinib have been identified. The most notable side effect was mild acne.20 After presenting this research, questions were asked about the safety profile of upadacitinib for patients with a history of cancer. However, researchers advised that there is not enough long-term data to offer guidance in this regard.18
TOPICAL JAK INHIBITORS
Along with injectable and oral medications, topical JAK inhibitors are also in various trial stages. Delgocitinib cream is a pan-JAK inhibitor that completed a phase 2b study on eczema itch and pain.21 A total of 258 adults with chronic hand eczema were randomized into five groups, each advised to apply the cream twice per day. Delgocitinib creams varied by strengths which were 20 mg/g, 8mg/g, 3 mg/g, 1 mg/g, and a vehicle cream. Participants receiving the highest strength cream experienced significant reductions in itch and pain within one week. All groups using delgocitinib cream saw more significant improvements than those given the vehicle cream.21
The results of two phase 3 studies on ruxolitinib cream (which is selective for JAK 1 and JAK 2) demonstrated positive outcomes for patients ages 65 and older, specifically for sleep disturbance and pruritis.22 In a double-blind study lasting over two years, 1249 patients ages 12 and older were randomized to receive 0.75% ruxolitinib cream, 1.5% ruxolitinib cream, or vehicle cream twice a day for eight weeks.23 There were 114 adults over age 65 analyzed as a subgroup. The anti-inflammatory effects and quality of life improvements are encouraging for an under-researched population of older adults with AD.22 Data from these studies helps confirm the benefits of ruxolitinib cream as previously described in the TRuE-AD studies.23
PSYCHOSOCIAL ASPECTS OF AD IN CHILDREN, CAREGIVERS, AND PROVIDERS
The AD-GAP (Atopic Dermatitis - Global Adolescent & Pediatric) study evaluated the qualitative and quantitative experiences of children and adolescents living with AD, along with their parents or caregivers and physicians. Researchers identified distinct disconnections between the perceptions held by young patients affected by AD and others involved in their care.3
A qualitative phase of the study, between May through September 2020, used short questionnaires, comprehensive interviews, and emotional response tests (age-appropriate images to elicit discussions about feelings) to gather research on the psychosocial impact of AD on kids and teens.3 In addition, investigators evaluated a sample of 72 caregivers and 72 physicians (including pediatricians, dermatologists, and allergists). Later, from March through April 2021, investigators conducted a quantitative study phase with 1447 patients, 1447 caregivers, and 1092 physicians, 45% of whom were dermatologists.3
Key findings were as follows:
- Children and teens tended to focus on the present.
- Teens described their condition as painful, itchy, and annoying, saying they prefer to be alone and cannot engage in activities they enjoy.
- Parents and caregivers showed concern about the future with AD and how it might affect their child in the years to come, namely their career and romantic relationships.
- When asked about their concerns over treatment side effects, 68% of caregivers reported worrying about the impact that AD treatment may have on their child, and 63% feared that the condition would worsen as they get older.3
- Physicians often reported poor patient compliance and admitted to not addressing quality of life at each visit due to barriers like time constraints or avoidance of sensitive topics.3
A thought-provoking discussion followed with the chair, Korey Capozza, offering a unique perspective as the parent of an adolescent with AD.24 Practical strategies for providers included:
- Utilize time in the waiting room with established survey tools to open up deeper discussions during appointments
- Separate parents and adolescents during visits to navigate around power struggles and unproductive family dynamics
- Facilitate young patient independence and accountability over the management of AD by encouraging teens and older children to lead the discussion and participate in decision-making
- Ensure that families meet with the same providers consistently, when possible, to build a bond
- Acknowledge the process of eliminating old coping mechanisms as treatments become effective and potentially transform patients' lives
JAK INHIBITION BEYOND AD
This year's EADV Congress also discussed how novel JAK inhibitors offer potential treatment options for multiple dermatological conditions.
For example, a multicenter phase 2 study on a small molecule JAK inhibitor, Jaktinib, shows early promise for severe alopecia.25 This three-arm, open-label trial randomized 111 adult participants to receive 200 mg of Jaktinib per day, 150 mg per day, or 50 mg twice daily. All groups saw significant benefits at 24-weeks. In addition, lower doses proved effective with fewer side effects, suggesting excellent potential for future studies.
Adults with non-segmental vitiligo should keep an eye out for an oral JAK 3/TEC inhibitor called ritlecitinib (PF-06651600). A phase 2b trial of 364 adult patients found the drug effective and well-tolerated compared to placebo.26 Varying doses among six groups showed continuous improvements through 48 weeks of treatment. However, the steepest changes were observed during weeks 24 to 48 when all participants were given the higher dose of 200 mg for weeks 24 to 28, followed by 50 mg maintenance through week 48.26
CONCLUSIONS
Patients with AD now have further treatment options, as oral JAK inhibitors abrocitinib, baricitinib, and upadacitinib continue to gain approval in new countries.2 The combination of older and newer therapies stands to benefit the growing needs of patients with a range of inflammatory conditions in dermatology and beyond. Compared to just three publications in the 1940s, today, over 2000 studies have been conducted on AD, and over 1000 trials are currently underway.27 Having a choice of numerous oral medications, topical creams, and subcutaneous treatments will better equip providers with targeted therapies to suit each patient's unique biological and lifestyle needs.28
Questions for Reflection:
1. How has the options for the treatment of atopic dermatitis (AD) changed? What has driven the revolution in this field?
2. In your opinion, in what way would the evolving treatment paradigm impact how we manage and treat our patients with AD in the future?
3. How can we maximize the use of currently available treatments for our patients with AD?
4. Based on emerging research and study results, where do biologics and JAK inhibitors fit into the treatment landscape for AD? Which patient do you think will benefit the most from these treatments?

References:
1. Guttman-Yassky E. Efficacy and safety results of KHK4083/AMG 451 (anti-OX40 mAb) in subjects with moderate to severe atopic dermatitis: a phase 2, multicentre, randomized, double-blind, parallel-group, placebo-controlled study. Presented at EADV 2021; Presentation ID D3T01.1B.
2. Nezamololama N, Fieldhouse K, Metzger K, Gooderham M. Emerging systemic JAK inhibitors in the treatment of atopic dermatitis: a review of abrocitinib, baricitinib, and upadacitinib. Drugs in Context 2020; 9: 2020-8-5. DOI: 10.7573/dic.2020-8-5
3. Paller A. Addressing the full impact of AD. Presented at EADV 2021; Presentation ID SAT 51.02.
4. Guttman-Yassky E. AD pathophysiology and role of IL-1α in AD. Presented at EADV 2021; Presentation ID SAT 59.02.
5. Irvine A. Overview of therapeutic targets in AD. Presented at EADV 2021; Presentation ID SAT 59.03.
6. Etrasimod, a novel, oral selective sphingosine 1-phosphate receptor modulator, in adults with moderate-to-severe atopic dermatitis: analysis of patient-reported outcomes and itch in a phase 2 clinical trial. Abstract Number: 1293
7. Weidinger S. A Phase 2a study of KY1005, a novel non-depleting anti-OX40Ligand (OX40L) mAb in patients with moderate to severe AD. Presented at EADV 2021; Presentation ID D1T01.3A.
8. Clinical safety and efficacy of RPT193, an oral CCR4 inhibitor: results from a randomized, placebo-controlled Phase 1b monotherapy trial in patients with moderate-to-severe atopic dermatitis. Abstract Number: 2746
9. Bissonnette R. Efficacy and safety of spesolimab, an anti-interleukin-36 receptor antibody, in patients with moderate-to-severe atopic dermatitis. Presented at EADV 2021; Presentation ID FC01.06.
10. Reich K. Efficacy and safety of abrocitinib versus dupilumab in adults with moderate-to-severe atopic dermatitis who received background topical therapy in a 26-week, randomized, head-to-head trial. Presented at EADV 2021; Presentation ID D3T01.2B.
11. Predictors of maintained response with tralokinumab every four weeks dosing in adults with moderate-to-severe atopic dermatitis. Abstract Number: 2948
12. Blauvelt A. Two-year maintenance of response with tralokinumab in moderate-to-severe atopic dermatitis: Interim analysis of the ECZTEND open-label extension trial. Presented at EADV 2021; Presentation ID FC01.04.
13. Bruin‑Weller M. Long-term efficacy of dupilumab in adults with moderate-to-severe atopic dermatitis: Results from an open-label extension trial up to 172 weeks. Presented at EADV 2021; Presentation ID FC01.02.
14. Long-term efficacy of dupilumab in adults with moderate-to-severe atopic dermatitis: results from an open-label extension trial up to 172 weeks. Abstract Number: 2008
15. Simpson E. Efficacy of abrocitinib in severe and difficult-to-treat patients with moderate-to-severe atopic dermatitis in the phase 3 JADE COMPARE study. Presented at EADV 2021; Presentation ID FC01.05.
16. Efficacy of abrocitinib in severe and difficult-to-treat patients with moderate-to-severe atopic dermatitis in the phase 3 JADE COMPARE study. Abstract Number: 1024
17. The impact of abrocitinib on vaccine-induced immune responses in adolescents with moderate-to-severe atopic dermatitis undergoing routine tetanus, diphtheria, and pertussis vaccination in phase 3 JADE TEEN. Abstract Number: 794
18. Blauvelt A. Efficacy and safety of switching from dupilumab to upadacitinib in moderate-to-severe atopic dermatitis: results from an open label extension trial. Presented at EADV 2021; Presentation ID D1T01.3B.
19. Efficacy and safety of upadacitinib vs dupilumab treatment for moderate to severe atopic dermatitis in four body regions - analysis from the Heads Up study. Abstract Number: 1816
20. Thyssen J. Efficacy and safety of upadacitinib vs dupilumab treatment for moderate to severe atopic dermatitis in four body regions - Analysis from the Heads Up study. Presented at EADV 2021; Presentation ID FC01.03.
21. Agner T. The topical pan-JAK inhibitor delgocitinib in a cream formulation reduces itch and pain in chronic hand eczema. Presented at EADV 2021; Presentation ID FC06.05.
22. Szepietowski J. Efficacy and safety of ruxolitinib cream among patients aged ≥65 years with atopic dermatitis: Pooled results from two phase 3 studies. Presented at EADV 2021; Presentation ID FC01.01.
23. Efficacy and safety of ruxolitinib cream among patients aged ≥65 years with atopic dermatitis: pooled results from two phase 3 studies. Abstract Number: 1092
24. Capozza K, et al. Living in the now with AD: panel discussion. Presented at EADV 2021; Presentation ID SAT 51.03.
25. Jaktinib, a novel JAK inhibitor in treatment of patients with severe alopecia areata: a randomized, three-arm, open-label and multicenter phase II dose-ranging study. Abstract Number: 2499
26. Efficacy and safety of the oral Janus kinase 3/TEC inhibitor ritlecitinib (PF06651600) in adults with active non-segmental vitiligo: results from a phase 2b, randomized, dose-ranging study with an extension period. Abstract Number: 2850
27. Langley R. Biologics and small molecules for atopic dermatitis. Presented at EADV 2021; Presentation ID D2T01.4C.
28. Salvati L, Cosmi L, Annunziato F. From emollients to biologicals: targeting atopic dermatitis. IJMS. 2021;22(19):10381. DOI: 10.3390/ijms221910381
This learning resource has been supported through educational funding from Pfizer Canada Inc. The content and views expressed in this resource are those of the faculty and do not necessarily reflect those of the publisher or sponsor. Physicians should consult federally accepted product monographs before making any diagnosis or treatment based on recommendations made in this document.