
Question 1: How does a patient’s A1C level influence the choice of DPP-4 inhibitor vs. SGLT2 inhibitor as add-on antihyperglycemic therapy?
Question 2: What is the importance of a patient’s age when choosing an add-on antihyperglycemic therapy?
Introduction
Metformin is the guideline-recommended first-line antihyperglycemic agent for individuals with type 2 diabetes mellitus (T2DM) in Canada.1 For patients who do not achieve their target A1C with metformin, the Diabetes Canada 2018 guidelines recommend selecting add-on therapy based on individual patient characteristics. There are clear recommendations for T2DM patients with clinical cardiovascular (CV) disease, in whom the guidelines recommend choosing one of the three agents with proven CV benefit: empagliflozin, liraglutide or canagliflozin.1 For patients without CV disease, the guidelines prioritize a DPP-4 inhibitor, GLP-1 receptor agonist or SGLT2 inhibitor if avoidance of hypoglycemia and/or weight gain is a priority.
The American Association of Endocrinologists and American College of Endocrinology's recommendations list GLP-1 receptor agonists as the preferred add-on to metformin, followed by SGLT2 inhibitors and DPP-4 inhibitors as preferred oral add-on options.2 As shown in Table 1, there are several important reasons why oral options such as SGLT2 inhibitors or DPP-4 inhibitors should be considered.3
In this review, I will answer some key questions focused on important variables that can help clinicians and their patients decide between DPP-4 inhibitors and SGLT2 inhibitors or both when considering oral add-on antihyperglycemic agent therapy in patients with T2DM who do not have CV disease.
Question 1: How does a patient’s A1C level influence the choice of DPP-4 inhibitor vs. SGLT2 inhibitor as add-on antihyperglycemic therapy?
ANSWER: Meta-analyses evaluating the A1C-lowering impact of adding a DPP-4 inhibitor or SGLT2 inhibitor to metformin have shown either no significant differences between the two classes or a small difference (0.11% to 0.17%) in favour of SGLT2 inhibitors.4-6
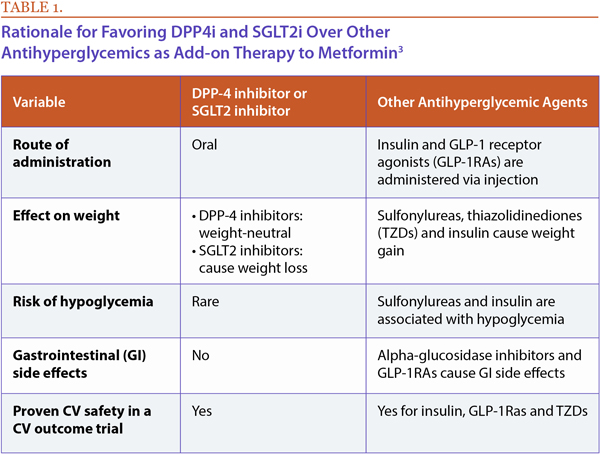
While these overall results do not lend themselves to conclusive recommendations, there are some important observations that can be made when one examines the glycemic efficacy by baseline A1C.3 Although no studies have prospectively stratified patients by baseline A1C in evaluation of the A1C-lowering efficacy of a DPP-4 inhibitor or SGLT2 inhibitor, post-hoc subgroup analyses have shown that the efficacy of SGLT2 inhibitors is greater than that of DPP-4 inhibitors among patients with higher baseline A1C (≥ 8-8.5%), while patients with baseline A1C at the lower end of the spectrum (< 8-8.5%) had numerically greater A1C benefit from DPP-4 inhibitors than from SGLT2 inhibitors (Figure 1).7,8
These findings suggest that, for patients whose A1C is further above target despite metformin therapy, an SGLT2 inhibitor might be reasonably favored over a DPP-4 inhibitor. In contrast, those whose baseline A1C is more modestly elevated might benefit more from adding a DPP-4 inhibitor than an SGLT2 inhibitor.
It is worth noting, however, that while SGLT2 inhibitor add-on therapy may be better than a DPP-4 inhibitor for A1C reduction among patients with markedly elevated A1C, evidence has shown that the combination of the two agents (in addition to metformin) is associated with significantly better A1C-lowering efficacy with greater likelihood of achieving A1C target than the addition of either agent alone. Three separate studies have evaluated the A1C-lowering impact of these classes alone or in combination with each other when added to metformin.7-9 In each study, patients who received an agent from both classes in addition to metformin were significantly more likely to achieve their A1C target compared to those who received the addition of an agent from either class alone. Across the three studies with baseline A1C levels in the 8.0% to 8.9% range, the proportion of patients who were able to reach A1C < 7.0% with the combination addition ranged from 41% to 62%; compared to 18% to 36% with DPP-4 inhibitor monotherapy or 22% to 33% with SGLT2 inhibitor monotherapy. In one study, with baseline A1C < 8.0%, single add-on therapy with a DPP-4 inhibitor allowed 58% of patients to achieve an A1C target of < 7.0%, whereas SGLT2 inhibitor add-on was only associated with 42% achieving A1C target. In contrast, with baseline A1C ≥ 8.0%, single add-on therapy with either a DPP-4 inhibitor or SGLT2 inhibitor was associated with no more than 19% of patients achieving target, whereas dual add-on therapy was associated with 50% of subjects achieving target (among those with baseline A1C 8.0% to < 9.0%) or 24% achieving target (among those with baseline A1C ≥ 9.0%).7 Therefore, consider DPP-4 inhibitor add-on when A1C is < 8.0%, but dual DPP-4 inhibitor plus SGLT2 add-on when baseline A1C is ≥ 8.0%.
Question 2: What is the importance of a patient’s age when choosing an add-on antihyperglycemic therapy?
ANSWER: There is ample evidence to suggest that patient age is a helpful factor to consider in choosing between DPP-4 inhibition and SGLT2 inhibition as an add-on to metformin therapy. The DPP-4 inhibitor class has demonstrated in several different studies that the expected reduction in A1C is not markedly different for older or younger adults (approximate placebo-corrected reduction of 0.6% to 0.7% among patients aged ≥ 65 years with baseline A1C of ~8.0%).10-13 Additionally, the side-effect profile of DPP-4 inhibitors in the older population is quite benign, essentially indistinguishable from that of placebo.
In contrast, the A1C-lowering efficacy of the SGLT2 inhibitors appears to be less robust in older adults than it is in younger patients.14,15 The approximate placebo-corrected A1C reductions associated with these agents among those aged ≥ 65 years is 0.4 to 0.6%. It has been hypothesized that this is due to two major factors: lower baseline A1C in the older patients included in the clinical trials; and reduced eGFR in the older cohorts compared to younger adults. Elderly patients may also be more prone to the volume-related side-effects of SGLT2 inhibition.
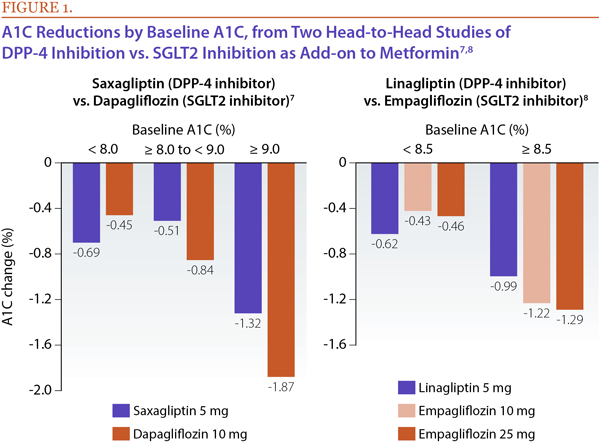
Kidney function is an important consideration in managing T2DM, because approximately 40% of patients with T2DM have chronic kidney disease (CKD). Measured by estimated glomerular filtration rate (eGFR), kidney function has been shown to be a predictor of A1C-lowering effects of SGLT2 inhibitors, with those with lower eGFRs (reduced kidney function) demonstrating less robust A1C lowering than those with preserved kidney function (Figure 2).16 This can be explained by the fact that efficacy of SGLT2 inhibitors in lowering A1C is dependent on the degree of glucosuria; as eGFR falls, there is less glucosuria in patients treated with SGLT2 inhibitors, and hence a drop in antihyperglycemic efficacy. SGLT2 inhibitor-treated patients with moderate renal impairment also may have more volume-related adverse effects (e.g., hypotension).
In contrast, clinical studies of DPP-4 inhibitors in patients with renal impairment show the same efficacy and safety as observed in those with normal renal function.17 Of note, the DPP-4 inhibitors alogliptin, saxagliptin and sitagliptin are excreted via the kidney, and pharmacokinetic studies demonstrate higher drug exposure in moderate to severe renal impairment, necessitating lower doses in these patients. This “renal dosing” is a pharmacokinetic issue and not related to efficacy or safety concerns; DPP-4 inhibitors are not considered nephrotoxic.
The Diabetes Canada guidelines and product monographs provide clear recommendations for the use of SGLT2 inhibitors and DPP-4 inhibitors in patients with reduced renal function.1 Canagliflozin should not be initiated in patients with an eGFR < 60 mL/min. If eGFR falls below 60 mL/min, canagliflozin can be continued at a dose of 100 mg, but it becomes contraindicated with eGFR < 45 mL/min. However, canagliflozin can be considered for CV and renal protection in those with eGFR > 30 mL/min and clinical CV disease according to Diabetes Canada.1 Dapagliflozin is contraindicated in patients with an eGFR < 60 mL/min. The updated product monograph for empagliflozin allows for initiation with an eGFR ≥ 30 mL/min and the agent is contraindicated with an eGFR < 30 mL/min.18 DPP-4 inhibitors, as mentioned above, can be used safely in patients with renal impairment. Linagliptin (not renally excreted) can be prescribed at the usual dose of 5 mg, but should be used with caution in patients with ESRD due to a lack of clinical data. Alogliptin, saxagliptin and sitagliptin each require renal dosing in moderate and severe renal impairment. Saxagliptin is not recommended in patients with ESRD, while alogliptin and sitagliptin can be used at doses of 6.25 mg and 25 mg respectively.
Taken together, these efficacy and safety observations suggest that, for older patients without CV disease with T2DM who are not at A1C target despite metformin monotherapy, it is reasonable to preferentially recommend a DPP-4 inhibitor rather than an SGLT2 inhibitor as add-on therapy.
References:
1. Lipscombe L, Booth G, Butalia S, et al. Diabetes Canada 2018 clinical practice guidelines for the prevention and management of diabetes in Canada: pharmacologic glycemic management of type 2 diabetes in adults. Can J Diabetes 2018; 42(Suppl 1):S88-S103.
2. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2018 executive summary. Endocr Pract 2018; 24(1):91-121.
3. Goldenberg RM. Choosing dipeptidyl peptidase-4 inhibitors, sodium-glucose cotransporter-2 inhibitors, or both, as add-ons to metformin: patient baseline characteristics are crucial. Clin Ther 2017; 39(12):2438-47.
4. Mishriky BM, Tanenberg RJ, Sewell KA, et al. Comparing SGLT-2 inhibitors to DPP-4 inhibitors as an add-on therapy to metformin in patients with type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab 2018; 44(2):112-20.
5. Wang Z, Sun J, Han R, et al. Efficacy and safety of sodium-glucose cotransporter-2 inhibitors versus dipeptidyl peptidase-4 inhibitors as monotherapy or add-on to metformin in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes Metab 2018; 20(1):113-20.
6. Maruthur NM, Tseng E, Hutfless S, et al. Diabetes mmedications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2016; 164:740-51.
7. Rosenstock J, Hansen L, Zee P, et al. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care 2015; 38:376-83.
8. DeFronzo RA, Lewin A, Patel S, et al. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care 2015; 38:384-93.
9. Pratley RE, Eldor R, Raji A, et al. Ertugliflozin plus sitagliptin versus either individual agent over 52 weeks in patients with type 2 diabetes mellitus inadequately controlled with metformin: The VERTIS FACTORIAL randomized trial. Diabetes Obes Metab 2018; 20(5):1111-20.
10. Barnett AH, Huisman H, Jones R, et al. Linagliptin for patients aged 70 years or older with type 2 diabetes inadequately controlled with common antidiabetes treatments: a randomised, double-blind, placebo-controlled trial. Lancet 2013; 382:1413-23.
11. Barzilai N, Guo H, Mahoney EM, et al. Efficacy and tolerability of sitagliptin monotherapy in elderly patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Curr Med Res Opin 2011; 27:1049-58.
12. Doucet J, Chacra A, Maheux P, et al. Efficacy and safety of saxagliptin in older patients with type 2 diabetes mellitus. Curr Med Res Opin. 2011; 27:863-9.
13. Pratley RE, McCall T, Fleck PR, et al. Alogliptin use in elderly people: a pooled analysis from phase 2 and 3 studies. J Am Geriatr Soc 2009; 57:2011-9.
14. Bode B, Stenlof K, Harris S, et al. Long-term efficacy and safety of canagliflozin over 104 weeks in patients aged 55-80 years with type 2 diabetes. Diabetes Obes Metab 2015; 17:294-303.
15. Leiter LA, Cefalu WT, de Bruin TW, et al. Dapagliflozin added to usual care in individuals with type 2 diabetes mellitus with pre-existing cardiovascular disease: a 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. J Am Geriatr Soc 2014; 62:1252-62.
16. Cherney DZ, Cooper ME, Tikkanen I, et al. Pooled analysis of Phase III trials indicate contrasting influences of renal function on blood pressure, body weight, and HbA1c reductions with empagliflozin. Kidney Int 2018; 93(1):231-44.
17. Davis TM. Dipeptidyl peptidase-4 inhibitors: pharmacokinetics, efficacy, tolerability and safety in renal impairment. Diabetes Obes Metab 2014; 16(10):891-9.
18. JARDIANCE product monograph. Boehringer Ingelheim Canada Ltd. April 16, 2018.
Development of this article was made possible through the financial support of Merck Canada Inc. The opinions expressed herein are those of the author, and do not necessarily reflect the views and opinions of Merck Canada Inc. The author had complete editorial independence in the development of this article and is responsible for its accuracy. The sponsor exerted no influence in the selection of content or material published.