

In ensuring we do no harm, as physicians treating bipolar disorder we need to consider both the mental and physical health of the patients we are treating. Understanding the impact of treatments both on short-term symptom control and long-term illness prevention is an integral part of balancing the management of this disease. In regards to bipolar depression, it is especially important to consider the physical, or somatic, comorbidities that can accompany the disorder, including obesity, diabetes and cardiovascular disease, as these are common among patients and their exacerbation often increases rates of disease recurrence and relapse rates as well as patient non-compliance.1-3 Therapies selected for the treatment of bipolar disorder, when possible, should not add to this burden and should instead be metabolically friendly to patients. This requires a sophisticated approach to prescribing and an integrated model of caring for patients, which will ultimately result in better overall long-term outcomes. When we treat the mind and respect the body, we can target the disease while mitigating the negative physical health outcomes that can accompany pharmacotherapy.
Comorbidities and the Burden of Disease
The decree to do no harm is embedded in the core tenets of medicine. However, this seemingly straightforward mandate can quickly become complicated when we are tasked with treating one chronic illness using a therapy that increases the risk of another chronic illness. This is especially evident in the treatment of bipolar depression.
To understand why we need to prioritize cardiometabolic issues in individuals with bipolar depression, it is important to understand the differing concepts of comorbidity and multimorbidity. One way to conceptualize this is to define the “index disease” as the main condition being targeted, while defining “comorbidity” as a medical condition that exists at the time of diagnosis (or post diagnosis) but is not a consequence of the index disease. In contrast, “multimorbidity” can be described as the concurrent existence of two or more chronic diseases.4
Viewing bipolar depression through this lens, it is known that even prior to the diagnosis of a mood disorder, individuals who have never been treated for depression have higher rates of obesity and are overweight compared to the general population.5 It is not yet known whether depression precedes weight gain or vice-versa, but the link between them may involve inflammation.6,7 It is known that increased weight impacts the index bipolar disease by increasing rates of recurrence in treated patients2 and rates of relapse secondary to non-compliance.3

Increased weight itself also brings with it an elevated risk of diabetes, metabolic syndrome and cardiovascular events.3 It is estimated that the incidence of metabolic syndrome in bipolar disorder is between 20-66%, approximately two-fold higher than that in the general population.1 Cardiovascular disease has also been associated with a near doubling of risk when examined in studies that specifically assess cardiovascular mortality among patients with bipolar disorder compared to the general population.8 In patients with bipolar depression and in the general population, obesity has become an equal, if not greater, contributor to the burden of disease than smoking.9 Importantly, youth experiencing their first psychotic episode appear even more likely to experience medication-related weight gain,10 with evidence also suggesting they may be at increased risk for type 2 diabetes in association with psychotropic treatment.11 This observation highlights the importance of judicious prescribing practices and prevention strategies at the earliest stages of bipolar illness to mitigate the long-term harms to the physical body.
Metabolic adverse effects of pharmacotherapies may also be very concerning to our patients, contributing to perceived morbidity, quality-of-life reduction, and reduced satisfaction with care. In a multinational survey conducted among 1,300 people with bipolar disorder, weight gain was the medication-related adverse event most commonly cited by patients as having a negative impact on quality of life (Figure 1).12 Most respondents also identified weight gain and general physical health as topics that their healthcare providers did not address adequately, and most were not screened or monitored for medical risk factors and disorders.12
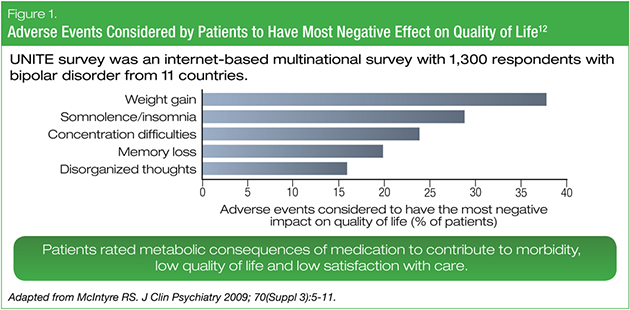
Regardless of whether the increased risks of metabolic syndrome or cardiovascular disease are multimorbid or comorbid with bipolar disorder, it is understood they are associated with decreased life expectancy and a worsening quality of life.13,14 These significant physical-health issues also interact in more subtle ways, as obesity has been linked to worse cognitive function among individuals with bipolar disorder13 and to the increased risk of dementia in the general population.14 The cardiometabolic adverse effects of pharmacotherapy not only affect our patients’ physical health but can also exacerbate their mental health issues.
Treatment Selection
Despite a wealth of evidence that highlights the risk of medication-induced cardiometabolic adverse effects, most people with bipolar disorder receive initial treatment that contradicts the guidelines for somatic health conditions, as well as the guidelines for their index psychiatric disorder.12 It is therefore important that we take into consideration potential metabolic effects and to avoid exacerbating weight gain when choosing a medication.
The most recent guidance for the acute treatment of bipolar depression in Canada comes from the 2013 Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines.15 These guidelines recommend the mood stabilizers lithium or lamotrigine, and the atypical antipsychotic (AAP) quetiapine, as first-line monotherapies for the treatment of acute bipolar depression. They recommend the mood stabilizer divalproex, and the AAP lurasidone, as second-line monotherapies. With respect to AAP’s, a more recent American consensus document (the 2015 Medicaid Guidelines), recommends quetiapine or lurasidone as monotherapy.16 When considering the two classes of agents, finding the right combination of efficacy and tolerability can be a challenge for physicians.
Treating the mental-health condition should be first and foremost in managing bipolar depression. Therefore, if contemplating prescribing an AAP, we should look to the most tolerable AAPs when considering treatment selection. When evaluating and comparing the tolerability profiles of various AAPs, useful metrics to consult are an agent’s number needed to treat (NNT) and its number needed to harm (NNH). A low NNT is desired, accompanied by a high NNH. In regards to pooled short-term weight gain versus placebo, lurasidone had the highest NNH of the AAPs, surpassing the other agents by a two- to three-fold margin (Figure 2).17
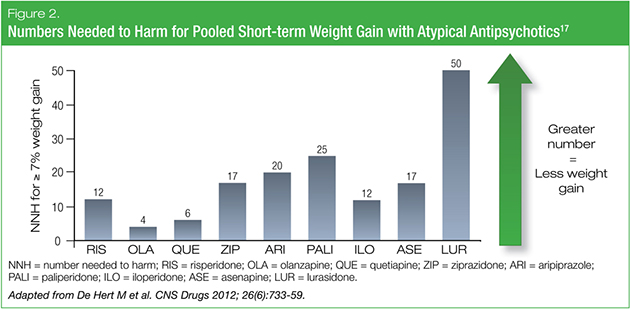
According to data obtained from the U.S. Food and Drug Administration, approved AAP pharmacotherapies for bipolar disorder all have single-digit NNTs (i.e., > 10% advantage over placebo), but the NNHs for adverse effects vary widely.18 Some highly efficacious agents are just as likely to yield adverse effects as therapeutic benefit, but they may be interventions of choice in more acute severe illness. In contrast, some less efficacious agents with better tolerability may be interventions of choice in more chronic mild-moderate illness. We may, therefore, have to start thinking beyond a “what makes you well keeps you well” approach to pharmacotherapy, and instead consider a more nuanced or “staged” approach to prescribing that balances acute efficacy with long-term tolerability. For the authors of the 2015 Medicaid Guidelines, for example, the metabolic hazards of olanzapine were seen to justify its recommendation as a Level 2B treatment for bipolar depression.16 This was not a reflection of the effectiveness of the therapy, but a ranking based on tolerability, adherence issues and a long-term view of patient quality of life.
In regards to Level 1 evidence demonstrated in randomized controlled clinical trials, quetiapine and lurasidone monotherapy have antidepressant efficacy in the acute treatment of bipolar I depression.19,20 Of the two, only quetiapine has been demonstrated as an efficacious treatment of bipolar II depression.21 However, lurasidone has a better metabolic profile (i.e., minimal weight gain and no clinically meaningful alterations in blood glucose or lipids)22 and can be used as adjunctive therapy in combination with lithium or divalproex.23 In the 2013 CANMAT guidelines, monotherapy with the antidepressants gabapentin, aripiprazole and ziprasidone, or adjunctive therapy with ziprasidone or levetiracetam, are not recommended.15
Screening and Monitoring Metabolic Risk
The first step in managing metabolic issues in patients with bipolar disorder is initiating the conversation. This issue can be sensitive and difficult to approach. One successful strategy involves utilizing “The 5 As” (ask, assess, advise, agree, and assist), a program developed for smoking cessation that can be adapted for obesity counseling. It involves 1) asking permission to discuss weight; 2) assessing weight and exploring drivers and complications of excess weight; 3) advising the patient about the health risks of obesity, the benefits of modest weight loss, the need for a long-term strategy and treatment options; 4) agreeing on realistic weight-loss expectations, targets, behavioral changes and specific details of the treatment plan; and 5) assistance in identifying and addressing barriers, providing resources, and arranging regular follow-up.24
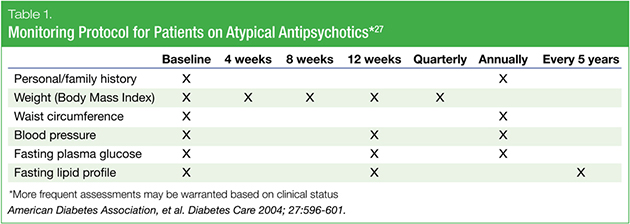
Since the “assistance” step is critical to the success of such a strategy—especially from the perspective of family physicians or psychiatrists—it is important for clinicians to feel comfortable counseling patients on health behaviors such as diet and exercise.25 Unique to addressing cardiometabolic issues in patients with bipolar disorder is the need to be very aware of the different risk profiles associated with current approved therapies.26 Due to the significant metabolic complications of various therapies, standard monitoring protocols have been developed. For example, in North America, the key metabolic monitoring guideline is the American Diabetes Association/American Psychiatric Association (ADA/APA) consensus document. This guideline specifies baseline and interval monitoring of blood glucose and lipid parameters, and a review of the patient’s medical history and physical measurements including weight, waist circumference, and blood pressure (Table 1).27
Summary
The priority for us as clinicians managing patients with bipolar disorder is to treat the index disease. However, we must think beyond treating the mind and also respect the body, as newer treatment options become available. While it is imperative that we treat bipolar depression with effective and targeted therapy, it is also crucial to consider the effects of that therapy, given the increased risk for diabetes, obesity and cardiovascular disease in this already-at-risk patient population. Medication-associated weight gain and metabolic syndrome not only contribute to decreased patient quality of life, but also to the burden of the index disease by compounding the symptoms of depression, making treatment more difficult. Therapies that maximize efficacy and minimize harm should be chosen on a case-by-case basis and paired with weight-management strategies that are easy to follow and welcomed by patients. If we are to treat the mind and respect the body, we need to understand the impact of therapies both on short-term symptom control and long-term overall health.
Development of this article was funded by Sunovion Pharmaceuticals Canada Inc. The authors had complete editorial independence in the development of this article and are responsible for its accuracy and completeness. Editorial assistance was provided by STA HealthCare Communications.
References
1. McIntyre RS, Danilewitz M, Liauw SS, et al. Bipolar disorder and metabolic syndrome: an international perspective. J Affect Disord 2010; 126(3):366-87.
2. Fagiolini A, Kupfer DJ, Houck PR, et al. Obesity as a correlate of outcome in patients with bipolar I disorder. Am J Psychiatry 2003; 160(1):112-7.
3. Lett TA, Wallace TJ, Chowdhury NI, et al. Pharmacogenetics of antipsychotic-induced weight gain: review and clinical implications. Mol Psychiatry 2012; 17(3):242-66.
4. Ording AG, Sørensen HT. Concepts of comorbidities, multiple morbidities, complications, and their clinical epidemiologic analogs. Clin Epidemiol 2013; 5: 199-203.
5. Taylor V, Macdonald K, McKinnon MC, et al. Increased rates of obesity in first-presentation adults with mood disorders over the course of four-year follow-up. J Affect Disord 2008; 109(1-2):127-31.
6. Capuron L, Su S, Miller AH, et al. Depressive symptoms and metabolic syndrome: is inflammation the underlying link? Biol Psychiatry 2008; 64(10):896-900.
7. Lemche E, Chaban OS, Lemche AV. Neuroendorine and epigentic mechanisms subserving autonomic imbalance and HPA dysfunction in the metabolic syndrome. Front Neurosci 2016; 10:142.
8. Weiner M, Warren L, Fiedorowicz JG. Cardiovascular morbidity and mortality in bipolar disorder. Ann Clin Psychiatry 2011; 23(1):40-47.
9. Jia H, Lubetkin EI. Trends in quality-adjusted life-years lost contributed by smoking and obesity. Am J Prev Med 2010; 38(2):138-44.
10. Correll CU, Manu P, Olshanskiy V, et al. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA 2009; 302(16):1765-73.
11. Galling B, Roldan A, Nielsen RE, et al. Type 2 diabetes mellitus in youth exposed to antipsychotics: a systematic review and meta-analysis. JAMA Psychiatry 2016; 73(3):247-59.
12. McIntyre RS. Understanding needs, interactions, treatment, and expectations among individuals affected by bipolar disorder or schizophrenia: the UNITE global survey. J Clin Psychiatry 2009; 70 Suppl 3:5-11.
13. Osby U, Brandt L, Correia N, et al. Excess mortality in bipolar and unipolar disorder in Sweden. Ach Gen Psych 2001; 58(9):844-50.
14. Morrison KM, Shin S, Tarnopolsky M, et al. Association of depression & health related quality of life with body composition in children and youth with obesity. J Affect Disord 2015; 172:18-23.
15. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord 2013; 15(1):1-44.
16. 2015 Florida Best Practise Psychotherapeutic Medication Guidelines for Adults. The University of South Florida, Florida Medicaid Drug Therapy Management Program 2015. Accessed online at: http://www.medicaidmentalhealth.org/_assets/file/Guidelines/Web_2015-Psychotherapeutic%20Medication%20Guidelines%20for%20Adults_Final_Approved1.pdf.
17. De Hert M, Yu W, Detraux J, et al. Body weight and metabolic adverse effects of asenapine, iloperidone, lurasidone and paliperidone in the treatment of schizophrenia and bipolar disorder: a systematic review and exploratory meta-analysis. CNS Drugs 2012; 26(6):733-59.
18. Ketter TA, Citrome L, Wang PW, et al. Treatments for bipolar disorder: can number needed to treat/harm help inform clinical decisions? Acta Psychiatr Scand 2011; 123(3):175-89.
19. Calabrese JR, Keck PE Jr., Macfadden W, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry 2005; 162(7):1351-60.
20. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry 2014; 171(2):160-8.
21. Ketter TA, Miller S, Dell’Osso B, et al. Balancing benefits and harms of treatments for acute bipolar depression. J Affect Disord 2014; 169 Suppl 1:S24-33.
22. McIntyre RS, Cha DS, Alsuwaidan M, et al. A review of published evidence reporting on the efficacy and pharmacology of lurasidone. Expert Opin Pharmacother 2012; 13(11):1653-9.
23. Tohen M, Vieta E, Calabrese J, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 2003; 60(11):1079-88.
24. Vallis M, Piccinini-Vallis H, Sharma AM, et al. Clinical review: modified 5 As: minimal intervention for obesity counseling in primary care. Can Fam Physician 2013; 59(1):27-31.
25. Lau D, Douketis JD, Morrison KM, et al. 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children. CMAJ 2007; 176(8 suppl):Online-1-117. Available at: http://www.cmaj.ca/content/suppl/2007/09/04/176.8.S1.DC1/obesity-lau-onlineNEW.pdf.
26. McIntyre RS, Cha DS, Alsuwaidan M, et al. A review of published evidence reporting on the efficacy and pharmacology of lurasidone. Expert Opin Pharmacother 2012; 13(11):1653-9.
27. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004; 27:596-601.