Considerations for perimenopausal and menopausal health are particularly important in today’s demographic environment; the age ranges with the largest populations of women in Canada are those spanning 50-54 years (approximately 1,300,000 individuals by Statistics Canada 2018 estimate) and 55-59 years (1,400,000).1 Vasomotor symptoms (VMS) of menopause are associated with significantly lower levels of health-related quality of life (HRQoL), and higher levels of work impairment and healthcare use.2 Increased rates of depression, anxiety, and joint stiffness are also common.2
Expert consensus guidelines, including those of the North American Menopause Society (NAMS), state that menopausal hormone therapy (MHT) is the most effective treatment for VMS, with additional potential benefits such as prevention of bone loss and improvement of genitourinary symptoms, sleep, wellbeing and/or HRQoL.3 The NAMS guidelines state that MHT has a favourable benefit:risk ratio for women younger than 60 years or within 10 years of menopause onset,3 and that therapy does not need to be routinely discontinued for those who require MHT after age 60.3
Much of this review focuses on a newer MHT agent: combined conjugated estrogens and bazedoxifene acetate (CE/BZA). This single daily dose tablet, which has been available in Canada since 2017, has been shown to provide relief from VMS, while offering a reassuring safety profile.4 In addition to a summary of clinical trial findings, we also share our personal clinical experience with CE/BZA.
It should be noted that some physicians and patients have lingering reservations about the safety of MHT. The key reasons for this, and an explanation of why these reservations are largely unfounded, are briefly explored below.
Background: The WHI, the Media and Inappropriate Misgivings About MHT
In 2002, the report of Women’s Health Initiative (WHI) study on combined estrogen + progestin MHT,5 together with subsequent sensationalized attention in the mainstream media, led many women (and many of their physicians and care providers) to conclude that there were unacceptable safety risks (e.g., increased breast-cancer risk) associated with combined MHT.5-11
It has been made clear over time, however, that it was inappropriate to extrapolate the findings of the WHI to the typical population of women who experience VMS of menopause.7-11 The WHI included women aged 50-79 years (average age 63 years). Only 33% were aged 50-59 years, those who most typically need and take MHT.3,5
In long-term follow-up of the WHI subgroup aged 50-59 years, combined MHT was not associated with any increase in risk of stroke, CHD or total mortality vs. placebo.8 In addition, first-time users of combined MHT in the entire study population showed no significantly increased risk for breast cancer.12 Furthermore, the WHI only investigated one type of estrogen (CE) combined with one type of progestin (MPA), delivered by one method (oral pill).5 Potential differences may exist between different types of MHT—including different formulations, routes, doses and administration—with respect to safety parameters.3 In summary, the external generalizability and extrapolating the WHI findings to women of all ages and to all types of MHT was not sound evidence-based medicine.
Conjugated Estrogens + Bazedoxifene Acetate (CE/BZA)
For women with a uterus and moderate-to-severe vasomotor symptoms, MHT consists of a systemic estrogen (to provide relief from VMS) and an endometrial protective agent (to reduce risk of endometrial hyperplasia that can occur with unopposed estrogen).3 The latter can be either a progestogen or BZA, a selective estrogen receptor modulator (SERM).3 When a SERM is combined with conjugated estrogens, this is known as a tissue-selective estrogen complex (TSEC).4 The Society of Obstetricians and Gynecologists of Canada has stated that a TSEC “will obviate the need for progestin co-therapy in women using systemic estrogen, both simplifying therapy and avoiding progestin-associated adverse effects.”13
Experience with the CE/BZA combination is extensive. A number of clinical trials of up to two years’ duration—the Selective Estrogens, Menopause, And Response to Therapy (SMART) studies—have evaluated CE/BZA among generally healthy, non-hysterectomized postmenopausal women.4 The combination also has been in clinical use in Canada since 2017 and in the U.S. since 2013.4,14
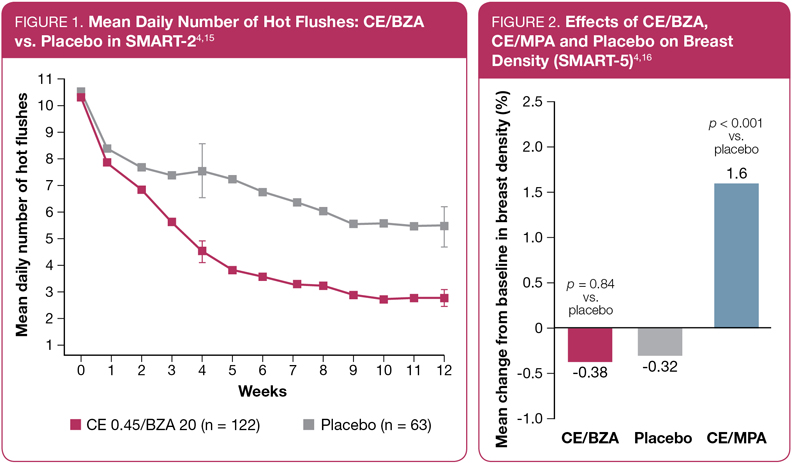
In the SMART-2 study, CE/BZA was associated with a significant reduction in the mean daily number of hot flushes (Figure 1), and in the average daily severity of hot flushes, compared to placebo.4,15 Significant differences in the number of hot flushes between CE/BZA and placebo were observed as early as week 3 (p = 0.008).15
CE/BZA also was associated with improvements in sleep and quality of life.4,15 In the SMART-2 study, for example, significant improvements vs. placebo were noted for time to fall asleep, sleep adequacy, sleep disturbance, and sleep problem index I and II.4,15
With respect to breast safety, CE/BZA has been shown to have a neutral effect on breast density when compared to placebo (Figure 2).4,16 In the same study, the active comparator arm (conjugated estrogens + medroxyprogesterone acetate [CEE/MPA], the same combination used in the WHI5) was associated with significantly increased breast density.4,16 (Note that the dose of CE was 0.45 mg in SMART-5 and 0.625 mg in WHI.) Breast pain was also not significantly different in comparisons of CE/BZA vs. placebo in the SMART trials (though breast-pain rates were higher in the CE/MPA group).4,15,17,18
Rates of endometrial hyperplasia were low with CE/BZA (< 1% over two years) and not significantly different from placebo.4,19 CE/BZA also was associated with amenorrhea rates similar to placebo. Cumulative amenorrhea at Year 1 of the SMART-1 trial was 83% for CE/BZA and 85% for placebo.4,20 Looking specifically at cycles 10-13, amenorrhea rates were > 93% for CE/BZA and placebo.20 Across the SMART studies, there was an overall low rate of discontinuations due to adverse events (7.5% for CE/BZA and 10% for placebo).4 Furthermore, there have been no significant safety signals detected during the post-marketing experience.
Clinical Experience with CE/BZA in Canada:
The Authors’ Perspectives
Dr. Denise Black
For women with a uterus and moderate-to-severe vasomotor symptoms, this is an innovative option. As with other estrogens, in post-marketing surveillance data from Canada and the U.S., the most frequently seen negative effect has been lack of complete control of VMS. The dose of estrogen (0.45 mg CEE) is considered low—roughly equivalent to 1 mg 17-beta estradiol or a 50 µg patch.21 When transitioning from higher-dose products, there may be worsening of flushing in up to 27% of women. For women having breast tenderness or troublesome bleeding with conventional EPT, more than 97% will have improvement in these symptoms by switching to TSEC.22
Post-marketing surveillance in Canada and the U.S. has not revealed any signals of increased venous thromboembolic or cardiovascular risk.
It is interesting to speculate on the breast effects of this combination in humans. Non-human primate data points to less breast proliferation compared to EPT.23 Whether or not this translates into fewer breast investigations, or a reduction in breast-cancer risk, remains to be seen. In the U.S., an ongoing National Cancer Institute clinical trial (PROMISE) is evaluating the effect of treatment with CE/BZA on biomarkers and gene expressions of breast proliferation in women with DCIS. Results are expected in 2022.24
Dr. Marla Shapiro
It is clear that treatment of women with VMS and intractable symptoms requires an individualized approach. The statement, “the lowest dose for the shortest duration,” has been misconstrued to mean “five years or less” of MH therapy. The NAMS Position statement points out that we now use the phrase, “the appropriate formulation, dose, and route of administration for the appropriate patient for the appropriate length of time to meet treatment goals,” underscoring the importance of this individualization of therapy.
In discussing hormonal treatment options with my patients, it is critical to have an understanding of their safety concerns along with the goals of therapy. In addition, many patients will express preference as to the route of administration, with some expressing the desire for a single-formulation therapy. It is clear that the initiation of a therapy, and persistence in taking that therapy, will be influenced by having a collaborative discussion with the patient.
The CE/BZA formulation has been a welcome addition to the choices of therapy a woman can be offered. For many women with moderate symptoms, this formulation will meet their expectations. However, for women with severe and frequent symptoms of VMS, the lower-dose formulations might not meet their expectations for what is considered a treatment success.
The novel use of a SERM as endometrial protection that is breast antagonist is also novel to our treatment armamentarium, and allows another option for women who will benefit from MHT.
Summary
When used for the appropriate population (i.e., women entering menopause, typically in their 50s), MHT is a safe, guideline-recommended first-line treatment for relief of VMS. CE/BZA is a combination therapy with extensive evidence of safety and efficacy, with a profile distinct from that of estrogen + progestogen. Its simple dosing (one strength, one tablet, once daily) makes it an appealing option for the treatment of VMS associated with menopause.
References:
1. Statistics Canada. Table 17-10-0005-01 Population estimates on July 1st, by age and sex. Available at: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000501. Accessed April 2019.
2. Whiteley J, daCosta DiBonaventura M, Wagner JS, et al. The impact of menopausal symptoms on quality of life, productivity, and economic outcomes. J Womens Health (Larchmt) 2013; 22(11):983-90.
3. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 2017; 24(7):728-53.
4. Pfizer Canada Inc. Duavive Product Monograph. January 16, 2019.
5. The Writing Group for the WHI Investigators. Risks and benefits of estrogen plus progestin in healthy post-menopausal women: Principal results of the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288(3):321-33.
6. Blake JM, Collins JA, Reid RL, et al. The SOGC statement on the WHI report on estrogen and progestin use in postmenopausal women. J Obstet Gynaecol Can 2002; 24(10):783-802.
7. Brown S. Shock, terror and controversy: how the media reacted to the Women’s Health Initiative. Climacteric 2012; 15:275-80.
8. Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007; 297:1465-77.
9. Langer RD, Manson JE, Allison M. Have we come full circle – or moved forward? The Women’s Health Initiative 10 years on. Climacteric 2012; 15:206-12.
10. Langer RD. The evidence for HRT: what can we believe? Climacteric 2017; 20:91-86.
11. Reid RL. Hormone therapy and breast cancer: risk communication and the ‘perfect storm’. Climacteric 2019; 22(1):13-16.
12. Chlebowski RT, Anderson GL, Gass M, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA 2010; 304(15): 1684-92.
13. Reid R, Abramson BL, Blake J, et al. Managing menopause. J Obstet Gynaecol Can 2014; 36(9):830-3.
14. Pfizer Inc. Duavee Prescribing Information (USA). September 2015.
15. Pinkerton JV, Utian WH, Constantine GD, et al. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause 2009; 16(6):1116-24.
16. Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens. A randomized controlled trial. Obstet Gynecol 2013; 121:959-68.
17. Lobo RA, Pinkerton JV, Gass ML, et al. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil Steril 2009; 92(3):1025-38.
18. Pinkerton JV, Harvey JA, Lindsay R, et al. Effects of bazedoxifene/conjugated estrogens on the endometrium and bone: a randomized trial. J Clin Endocrinol Metab 2014; 99(2):E189-98.
19. Pickar JH, Yeh IT, Bachmann G, et al. Endometrial effects of a tissue selective estrogen complex containing bazedoxifene/conjugated estrogens as a menopausal therapy. Fertil Steril 2009; 92(3):1018-24.
20. Archer DF, Lewis V, Carr BR, et al. Bazedoxifene/conjugated estrogens (BZA/CE): incidence of uterine bleeding in postmenopausal women. Fertil Steril 2009; 92(3):1039-44.
21. Lobo RA. The hope for KEEPS. Climacteric 2015 Apr; 18(2):108-9.
22. Kim SE, Lee DY, Choi D. Tissue-selective estrogen complex for women who experience breast discomfort or vaginal bleeding when on hormone therapy. Menopause 2019; 26(4):383-6.
23. Ethun KE, Wood CE, Register TC. et al. Effects of bazedoxifene acetate with and without conjugated equine estrogens on the breast of postmenopausal monkeys. Menopause 2012; 19(11):1242-52.
24. The PROMISE Study: Duavee in Women With DCIS. ClinicalTrials.gov Trial #NCT02694809.
Development of this article was made possible through the financial support of Pfizer Canada Inc. The opinions expressed herein are those of the authors, and do not necessarily reflect the views and opinions of Pfizer Canada Inc. The authors had complete editorial independence in the development of this article and are responsible for its accuracy. The sponsor exerted no influence in the selection of content or material published.