Introduction
Invasive meningococcal disease (IMD) is a potentially catastrophic infection that can cause serious, lifelong disabilities or even death within 24 hours of onset. It is relatively uncommon, but the initial nonspecific symptoms often lead to misdiagnosis and complications. The groups at highest risk for IMD are infants/toddlers and adolescents/young adults. Since the introduction of routine childhood immunization programs against serogroup C in 2002, incidence rates against this serogroup have declined significantly. Meningococcal serogroup B (MenB) is now the most predominant strain, accounting for more than 75% of infections in these groups. There are currently two vaccines available for protection against MenB (Bexsero® and TrumenbaTM). This review examines clinical-trial immunogenicity and safety data, as well as real-world surveillance data, for both vaccines.
Burden of Meningococcal Infection
IMD usually presents as an acute febrile illness with rapid onset of meningitis, septic shock, or both. Initial symptoms within the first 12 hours are nonspecific and may include fever, headache, sore throat, myalgia, fatigue, and decreased appetite.3 Over the next few hours, patients may develop signs and symptoms of meningitis, such as intense headache, photophobia, neck stiffness and drowsiness. After 16 hours, patients can exhibit purpuric or hemorrhagic rash, delirium, decreased level of consciousness, seizures, and circulatory collapse. Ten to 15 percent of cases are fatal, and death can occur within 24 hours.1,3
Among patients who survive the infection, 10-20% have long-term sequelae including neurologic dysfunction, hearing impairment, skin scarring/grafts, limb amputation, motor impairment, cardiovascular disease and renal impairment.1,2,4,5,6 Compared to well-matched control subjects, adolescent survivors of IMD report poorer mental health, social supports, educational outcomes and quality of life, and increased fatigue 18-36 months after IMD.7
Epidemiology of IMD in Canada
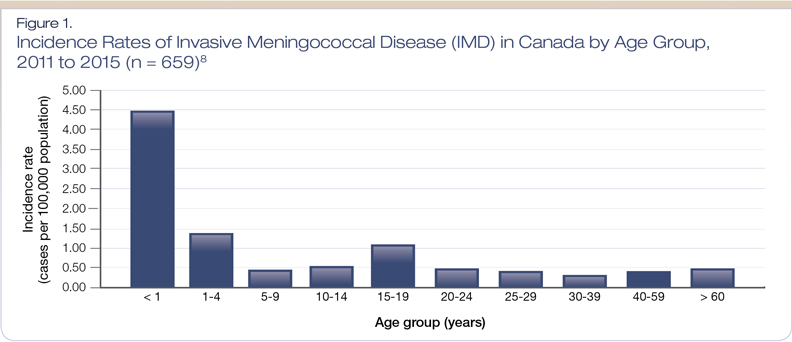
From 2011 to 2015, a total of 659 IMD cases were reported in Canada (median of 121 cases reported per year).8 Of these 659 cases, 75 were fatal. As shown in Figure 1, the highest incidence rates were among infants younger than one year, followed by toddlers and those aged 15-19 years.8
There is variability in meningococcal serogroup distribution depending on age. Among those aged 19 years or younger, serogroup B is by far the most common, accounting for 78% of cases of IMD.9 Other serogroups include serogroup Y (11%), serogroup C (5%) and serogroup W-135 (5%). In adults older than 19 years, serogroup B is still the most common (53%), followed by serogroup Y (26%), serogroup W-135 (11%), and serogroup C (9%).
There have been several outbreaks of IMD among adolescents and young adults in Canada. During the 2001 winter season in the Quebec City area, there were 44 confirmed cases of serogroup C IMD, with eight fatalities.10 At Acadia University in Nova Scotia in 2015, two students had confirmed serogroup B IMD, one of whom died.11,12 In 2017, British Columbia reported 11 confirmed cases of IMD among individuals aged 16-19 years in the interior of the province, five of which were serogroup W-135.13 There was one death among these 11 cases.
Risk Factors for IMD
Infants and toddlers are at the highest risk for IMD, due to their immature immune systems.14 Adolescents and young adults are at high risk due to their behavioural profile including sharing food, drinks, cosmetics, and smoking; increased social and physical contact; close-quartered living; and sports-related activities (e.g., water bottles, mouth guards).15-18
Other groups at high risk for IMD include those with complement, properdin, or factor D deficiencies; functional or anatomic asplenia; HIV infection; travellers to endemic areas; and those in certain occupations (e.g., laboratory workers, military personnel living in barracks).16,19-21
It should be noted that not all carriers of meningococcal bacteria develop symptoms. An estimated 10% of the overall population asymptomatically carries the bacteria in their nasopharynx and may transmit to others via respiratory droplets.22 This proportion is thought to be much higher among at-risk populations: approximately 25% of adolescents and young adults are thought to carry the bacteria, as are up to half of individuals living in crowded settings, such as university residences and military barracks.22,23
Meningitis Vaccines in Canada
There are seven meningococcal vaccines available in Canada: two monovalent vaccines targeting serogroup C (Menjugate® and NeisVac-C®), three quadrivalent vaccines targeting serotypes A, C, Y and W-135 (Menactra®, MenveoTM and Nimenrix®) and two vaccines selectively targeting serogroup B (Bexsero® and TrumenbaTM).24
The development of MenB vaccines has been challenging because the serogroup B polysaccharide is poorly immunogenic and structurally similar to human neural cell adhesion molecules, creating a potential for generation of autoimmune response.25 This challenge was overcome by targeting surface-exposed protein antigens, capable of inducing protective antibodies.
The multi-component MenB vaccine, Bexsero®, is indicated for the active immunization of individuals aged 2 months to 25 years against IMD caused by MenB.26 This vaccine contains four target antigens from N. meningitides serogroup B: Neisserial adhesin A (NadA), Neisseria Heparin Binding Antigen (NHBA), factor H binding protein (fHBP) subvariant 1.1 (subfamily B, B24), and porin A (PorA) protein.
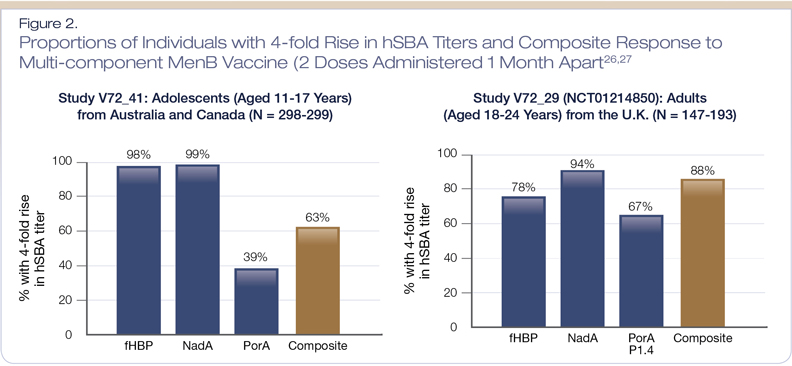
The safety and immunogenicity profile of this vaccine is based on data from 15 clinical studies, four of which were phase 3 studies that included adolescents and/or young adults.26 Figure 2 shows the immunogenicity results from a pivotal trial assessing the proportion of participants (aged 11-24 years) who had a four-fold rise from baseline in human serum bactericidal assay (hSBA) titers after receiving two doses of Bexsero, administered one month apart.26,27 Overall, the clinical trials demonstrated that Bexsero induced a robust immune response against MenB test strains following this two-dose vaccine schedule.27
Using data gathered from 17 million adults and children from 12 Canadian cities from 2006-2009, researchers assessed the potential strain coverage offered by Bexsero for 157 MenB strains.28 Overall, the predicted strain coverage was 66%.28
In clinical trials among adolescents and adults, the most frequent local and systemic adverse reactions after vaccination with Bexsero were pain, erythema, induration, malaise, headache and myalgia.26 The standard dosing schedule for Bexsero is two doses given at least four weeks apart.26
The other vaccine against invasive MenB disease, Trumenba, is a bivalent vaccine comprised of two recombinant lipidated fHBP variants and is indicated for active immunization to prevent IMD caused by MenB in individuals aged 10-25 years.29 The lipidated protein (the naturally occurring form of fHBP) induces antibodies that can kill MenB strains expressing fHBPs that are different from those in the vaccine, whereas non-lipidated variants are unable to induce broadly cross-reactive bactericidal responses.26 fHbp is an ideal target because it is expressed on > 95% of MenB strains and functions as an important immune evasion mechanism.30-32 fHbp is expressed as either subfamily A or subfamily B, with limited cross-reactivity between the two subfamilies. Subfamily B is the most common, accounting for about two thirds of fHBPs.
A survey of > 2,150 different invasive MenB isolates from 2000 to 2014 in 7 European countries, the United States and Canada demonstrated that > 91% of all MenB isolates expressed sufficient levels of fHbp to be susceptible to bactericidal killing by antibodies induced by Trumenba.33
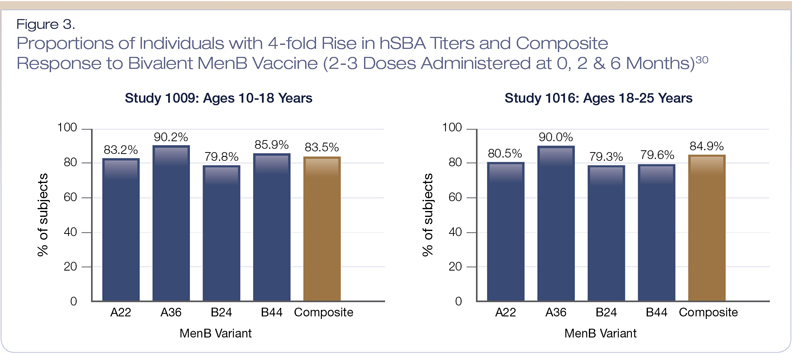
The immunogenicity and safety of Trumenba has been evaluated in 11 clinical trials involving more than 20,000 subjects.30 Three phase 3 clinical trials were conducted in children, adolescents and young adults (ages 10-26 years).30 Immunogenicity was assessed as the proportion of subjects who had a four-fold or greater increase from baseline in hSBA titers against each of the four strains, and the proportion of subjects who achieved a titer ≥ 1:8 (three strains) or 1:16 (one strain) for the four strains combined (composite response) after two or three doses. Overall, the vaccine showed robust immune responses in both age groups (10-18 years and 18-25 years), as > 80% had the predefined response (Figure 3).30
The most common adverse events were injection-site pain, which occurred in 76-87% of enrolled subjects.29 Other common adverse events (> 10% of subjects) included headache, fatigue, muscle pain, joint pain, chills, diarrhea and local-site swelling and redness.30
The standard dosing schedule for Trumenba is two doses, with the second dose administered six months following the first.30 For patients at increased risk of IMD, a three-dose schedule is recommended, with the second dose given at least one month after the first, and the third dose given four months after the second. Medical conditions causing increased risk of IMD include complement, properdin or factor D deficiencies, functional or anatomic asplenia (including sickle cell disease), certain genetic risk factors, and HIV infection.30
Conclusions
IMD is a very serious and potentially fatal infection, with a rapid onset and long-term sequelae for survivors. The groups at highest risk for IMD include infants and toddlers, adolescents and young adults, and those at increased risk because of underlying immunologic medical conditions and/or increased risk of exposure. Serogroup B is currently the predominant cause of IMD in Canada. Both available MenB vaccines have demonstrated robust immunogenicity profiles against circulating strains of MenB. It is important to discuss and recommend meningococcal vaccines with patients to optimize IMD-prevention strategies.
References:
1. Centre for Disease Control and Prevention. Manual for the Surveillance of Vaccine-preventable Diseases. Chapter 8: Meningococcal Disease. Available at: www.cdc.gov/vaccines/pubs/surv-manual/chpt08-mening.html. Accessed March 2019.
2. Rosenstein NE, Perkins BA, Stephens DS. Meningococcal disease. N Engl J Med 2001; 344(18):1378-88.
3. Thompson MJ, Ninis N, Perera R, et al. Clinical recognition of meningococcal disease in children and adolescents. Lancet 2006; 367(9508):397-403.
4. Bettinger JA, Scheifele DW, Le Saux N, et al. The disease burden of invasive meningococcal serogroup B disease in Canada. Pediatr Infect Dis J 2013; 32:e20-e25.
5. Viner RM, Booy R, Johnson H, et al. Outcomes of invasive meningococcal serogroup B disease in children and adolescents (MOSAIC): a case-control study. Lancet Neurol 2012; 11(9):774-83.
6. Brandtzaeg P. In: FroschM, et al (eds). Handbook of Meningococcal Disease: Infection Biology, Vaccination, Clinical Management. Weinheim, Germany: Wiley-VCH VerlagGmbH & Co, Weinheim, Germany, 2006, pp. 427-79.
7. Borg J, Christie D, Coen PG, et al. Outcomes of meningococcal disease in adolescence: prospective, matched-cohort study. Pediatrics 2009; 123(3):e502-9.
8. Public Health Agency of Canada. Vaccine Preventable Disease Surveillance Report to December 31, 2015. Available at: www.canada.ca/content/dam/phac-aspc/documents/services/publications/healthy-living/vaccine-preventable-disease-surveillance-report-december-31-2015/vaccine-preventable-disease-eng.pdf. Accessed March 2019.
9. Canadian Immunization Monitoring Program Active (IMPACT). fHBP Variant Diversity and Level of Surface Expression Among Invasive Neisseria meningitidis Serogroup B Isolates from Canada. Presented at International Pathogenic Neisseria Conference 2016.
10. Pinker S. Quebec’s $100-million campaign targets meningococcal disease. CMAJ 2001; 165(11):1520.
11. Communicable Disease Prevention & Control (2015) Meningococcal Meningitits. Available at: https://novascotia.ca/dhw/CDPC/meningitis.asp. July 2017.
12. CBC News. Acadia University dealing with “instututional outbreak” of meningitis. Available at: www.cbc.ca/news/canada/nova-scotia/acadia-university-dealing-with-institutional-outbreak-of-meningitis-1.2955992. March 2018.
13. B.C. Centre for Disease Control. Meningococcal vaccine offered to youth aged 15-19 across Okanagan region. Available at: www.bccdc.ca/about/news-stories/news-releases/2017/meningococcal-vaccine-offered-to-youth-aged-15-19-across-okanagan-region. December 2017.
14. Li YA, Tsang R, Desai S, et al. Enhanced surveillance of invasive meningococcal disease in Canada, 2006-2011. Can Commun Dis Rep 2014; 40(9):160-9.
15. World Health Organization. Meningococcal meningitis. Fact sheet no. 141. Available at: www.who.int/mediacentre/factsheets/fs141/en/. Accessed August 2017.
16. Harrison LH. Prospects for vaccine prevention of meningococcal infection. Clin Microbiol Rev 2006; 19(1):142-64.
17. Brigham KS , Sandora TJ. Neisseria meningitidis: epidemiology, treatment and prevention in adolescents. Curr Opin Pediatr 2009; 21(4):437-43.
18. Princeton University. Meningococcal Fact Sheet, October 2013. Available at: https://web. princeton.edu/sites/emergency/meningitis/Meningitis%20FAQ%2010-13.pdf. Accessed February 2018.
19. Bilukha OO, Rosenstein N, et al. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2005; 54(RR-7):1-21.
20. Memish ZA, Goubeaud A, Bröker M, et al. Invasive meningococcal disease and travel. J Infect Public Health 2010; 3(4):143-51.
21. Steffen R. The risk of meningococcal disease in travelers and current recommendations for prevention. J Travel Med 2010; 17(suppl):9-17.
22. Dwilow R, Fanella S. Invasive meningococcal disease in the 21st century – an update for the clinician. Curr Neurol Neurosci Rep 2015; 15(3):2.
23. Christensen H, May M, Bowen L, et al. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10(12):853-61.
24. Health Canada Drug Product Database. Available at: https://health-products.canada.ca/dpd-bdpp/index-eng.jsp. Accessed March 2019.
25. Finne J, Leinonen M, Mäkelä PH. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet 1983; 2(8346):355-7.
26. BEXSERO Product Monograph. GlaxoSmithKline Inc., August 2018.
27. Banzhoff A. Multicomponent meningococcal B vaccination (4CMenB) of adolescents and college students in the United States. Ther Adv Vaccines 2017; 5(1):3-14.
28. Bettinger JA, Scheifele DW, Halperin SA, et al. Diversity of Canadian meningococcal serogroup B isolates and estimated coverage of an investigational meningococcal serogroup B vaccine (4CMenB). Vaccine 2014; 32(1):124-30.
29. Nolan T, Santolaya ME, de Looze F, et al. Antibody persistence and booster response in adolescents and young adults 4 and 7.5 years after immunization with 4CMenB vaccine. Vaccine 2019; 37(9):1209-18.
30. TRUMENBA Product Monograph. Pfizer Canada Inc., July 2018.
31. McNeil LK, Murphy E, Zhao XJ, et al. Detection of LP2086 on the cell surface of Neisseria meningitidis and its accessibility in the presence of serogroup B capsular polysaccharide. Vaccine 2009; 27(25-26):3417-21.
32. Murphy E, Andrew L, Lee KL, et al. Sequence diversity of the factor H binding protein vaccine candidate in epidemiologically relevant strains of serogroup B Neisseria meningitidis. J Infect Dis 2009; 200(3):379-89.
33. Jiang HQ, Hoiseth SK, Harris SL, et al. Broad vaccine coverage predicted for a bivalent recombinant factor H binding protein based vaccine to prevent serogroup B meningococcal disease. Vaccine 2010; 28(37):6086-93.
Development of this article was made possible through the financial support of Pfizer Canada ULC. The opinions expressed herein are those of the authors, and do not necessarily reflect the views and opinions of Pfizer Canada ULC. The authors had complete editorial independence in the development of this article and are responsible for its accuracy. The sponsor exerted no influence in the selection of content or material published.