
In the June 2016 issue of the Canadian Journal of Diagnosis entitled, “What Kind of Depression Are You Treating? Diagnosing and Treating Bipolar Depression,” Dr. Roger McIntyre and his colleagues highlighted the importance of differentiating bipolar from unipolar depression. The failure to correctly identify the presence of a bipolar diathesis has a tremendous impact on long-term patient functioning and quality of life. However, treatment selection also has the potential to positively or negatively influence short-term and long-term quality of life. Once a diagnosis has been confirmed and a treatment plan has been formulated, clinicians should strive to avoid the potential cascade of negative physical health outcomes associated with some treatments. While many medications effectively treat the symptoms of mania and/or depression, they may also induce adverse effects that add to the burden of illness—particularly weight gain and metabolic syndrome. It is crucial that prescribers, in the midst of making acute treatment decisions, consider the long-term implications of their choices. This should include the physical health impact of medications, in addition to the efficacy and prophylactic benefits. Essentially, we must treat the mind while respecting the body.
Diagnostic Challenges
Bipolar disorder is commonly misdiagnosed, sometimes for decades,1,2 for a myriad of reasons.3,4 Most often, bipolar patients present to their healthcare provider during a depressive episode,5 which in cross-section often appears identical to uni-polar depression.5 A long-term study found that patients with bipolar I disorder were symptomatic for nearly one-half of the time throughout 13 years of follow-up. On average, those patients spent about 30% of their time depressed, while they were manic or hypomanic less than 10% of the time.6 A similar 13-year study of bipolar II patients found they were symptomatic for more than half the time, with depressive symptoms 54% of the time and hypomania only 1.3% of the study period.7
When patients with bipolar disorder are depressed, some do not recall or lack insight regarding their past manic or hypomanic episodes. Family history, which is helpful in establishing a bipolar diagnosis, is often murky, perhaps because the term “bipolar” has only recently been added to the lay lexicon. Patients often do not recognize that a family member might have a bipolar diagnosis, so clinicians must seek out a family story “suggestive” of bipolar disorder. This might include a family history of chronic depression and/or anxiety, suicide, substance abuse, “nervous breakdowns,” institutionalization, or marked family dysfunction or chaos.
Gathering collateral history is an essential element of making a bipolar diagnosis, particularly if a patient lacks insight regarding the severity of their illness. Lack of an adequate collateral history is sometimes due to the absence of an appropriate, accurate historian, but clinicians should seek out and encourage collateral historians.
Comorbidity is highly prevalent in bipolar disorder, further obscuring diagnostic clarity. Close to 90% of bipolar patients have a comorbid psychiatric disorder,8 and more than 50% have a medical comorbidity.9 The most common psychiatric comorbidities include: anxiety disorders (77%), behavioural disorders (54%) and substance use disorder (52%). The 2011 World Mental Health Survey8 found that while 20% of patients with unipolar depression have three or more psychiatric comorbidities, the rate was more than 60% for patients with bipolar disorder. The presence of comorbid mental illnesses often lead to more severe, sometimes treatment-resistant, symptoms, greater functional impairment, non-compliance and a heightened risk of suicide.10-13

Bipolar spectrum disorders (I, II and not otherwise specified [NOS]) are chronic, functionally impairing illnesses that affect about 4.5% of the population and have a profound impact on quality of life.14,15 When considering neuropsychiatric illnesses, the global disease burden associated with bipolar disorder, measured by productive time lost among working age adults, ranks second only to major depression.16 This statistic is not surprising, given the hefty toll of suicide associated with bipolar disorder. One-quarter of completed suicides may be related to bipolar disorder,17 which is associated with a 20-30 times greater suicide risk compared to the general population.18 Making the correct diagnosis as early as possible and developing an individualized treatment plan that includes effective, tolerable treatments and considers long-term adherence, should improve functional outcomes and maintain the best possible quality of life for patients. Characterizing the type of bipolar presentation, which is an important element of treatment selection, will further inform an effective clinical management plan that can minimize harms to the body without compromising treatment for the mind.
Treatment Selection Factors
There are many considerations when choosing a treatment for an acutely ill patient with bipolar disorder. These include patient/illness-specific, treatment-specific, and prescriber-specific factors. Clinicians must balance these sometimes competing factors when formulating a management plan that best suits their patient’s needs, both in the short term and long term.
Patient/illness-specific factors generally focus on current symptomatology, but clinicians should also consider how the patient has experienced their illness historically. When treating a first episode of illness, family history might provide valuable information and inform treatment choices. As well, the presenting symptoms of bipolar disorder may provide clues about the future course of illness. Most patients with bipolar disorder first present with depressive symptoms during their teen years, which may be preceded by or occur coincident with marked anxiety symptoms.19 If the first symptoms of bipolar disorder are mania or hypomania, this might foretell a more severe course of illness. Likewise, an earlier age of onset,20,21 the presence of rapid cycling,22 mixed features23 or comorbid conditions (e.g., attention deficit hyperactivity disorder [ADHD], substance abuse, a physical illness) might portend a more severe and difficult-to-treat illness.
For a patient with multiple previous episodes, issues like insight, likelihood of compliance, willingness to follow safety protocols, willingness to consider new treatment options, previous response to treatment, severity of past episodes, and suicidality must be considered. Additionally, the quality of social support, functional limitations (e.g., ability to maintain employment or stay in school), and engagement with care providers are essential considerations. Most importantly, a patient’s preferences are paramount.
A myriad of treatment-specific factors must also be considered when treating a patient with bipolar disorder. While most pharmacological treatments have empirical evidence for efficacy, studies do not provide information about the needs of individual patients. Thus, clinicians must employ a “trial and error” approach, which may be highly frustrating and disheartening for patients. Patients might lose faith in the process if an effective and tolerable treatment remains elusive for too long. Side effects are a major reason for non-compliance, particularly metabolic issues, excessive sedation, and sexual dysfunction. Drug-drug interactions must be considered if a patient is prescribed medications for other conditions. Ease of use (e.g., once daily dosing, depot drug delivery), cost and availability round out the extensive list of considerations.
Less often discussed are factors related to the treating clinician or care team. Prescriber knowledge and comfort is at the core of all pharmacological decisions. Some may prescribe based on habit rather than basing their decisions on an individual’s unique needs and preferences. Others may be hesitant to prescribe new treatment options, which may offer superior tolerability, perhaps due to lack of familiarity or confidence. Combination treatments for bipolar disorder are appropriate and increasingly employed because they often confer an additive benefit,24,25 yet some clinicians are reluctant to combine treatments, which may hamper symptomatic remission and full functional recovery. Many prescribers feel limited by sometimes-ill-informed provincial regulatory bodies, which may prevent clinicians from appropriately prescribing necessary, compassionate, sometimes life-saving treatments. An example is the apprehension some clinicians experience when prescribing benzodiazepines to bipolar patients for anxiety, agitation and sleep.
Hospital-based mental health providers are often under pressure to discharge patients as quickly as possible, leading to acute treatment choices that might have significant side effects, like weight gain or excessive sedation. When patients are discharged on these medications, their community healthcare provider may be loath to change acutely effective medication, fearful of provoking a relapse. Yet, they are then left to support and encourage a frustrated, disgruntled patient who hates their medication and will likely become non-compliant. Finally, an important barrier to adequate treatment is lack of access, particularly in remote areas where psychiatric support is limited.
Clinicians can challenge their usual practice, whether related to treatment selection or the development of treatment goals, by reviewing clinical trial evidence, gathering knowledge regarding approved drug indications, following published guidelines and engaging with colleagues to consider emerging practise experience. Critical goals of bipolar treatment are long-term mood stability and prophylaxis,26 and must take physical health outcomes into account. With the abundance of evidence available to highlight the negative impact of chronic, undertreated or untreated bipolar illness, it is imperative clinicians consider long-term management from the outset of treatment.2 With the length of untreated illness correlated with cognitive impairment, unemployment, poverty, social conflict and worsening overall physical health outcomes, early, effective, tolerable treatment that can safely and effectively transition from acute to long-term illness stability is the Holy Grail of bipolar management.

Historically, bipolar treatment has largely focused on the management of mania, perhaps due to the tremendous health and safety impact of mood elevation. The majority of Health Canada and Food and Drug Administration (FDA) approved medications for bipolar disorder are for the treatment of mania, a medical emergency that requires a rapid assessment of insight and safety and urgent medical intervention. However, recently, there has been a greater emphasis on the management of the depression pole, likely due to the preponderance of time most patients spend depressed,6,7 as well as the mortality associated with bipolar-related suicide.17,18
Perhaps because bipolar treatment has largely focused on managing or preventing mood elevation, clinicians have historically taken a short-term view of bipolar management. In their haste to rapidly control manic symptoms, clinicians sometimes fail to consider the long-term impact of their acute treatment choices. Additionally, patients, whether due to poor insight, lack of social support or a paucity of psychoeducation, rarely push clinicians to consider their long-term wellbeing when treatment is initiated.
Choice of Treatment
There is compelling scientific evidence regarding the efficacy of lithium, divalproex, and atypical antipsychotics (AAPs) for the treatment of mania.27,28 While lithium and valproate continue to be considered first-line treatment options for bipolar mania, some patients preferentially respond to one treatment or the other. Lithium responders tend to have a family history of bipolar disorder and experience classic euphoric mania. For patients who lack a bipolar family history, experience rapid cycling, mixed features, or substance abuse, valproate may be a better choice.27,28
The value of lithium for bipolar depression, as well as its robust long-term prophylactic benefits, has been established.29 Treating depression with valproate does not have similar clinical or empirical support. For some patients, lithium is a challenging medication to take in the long-term. Aside from its narrow therapeutic index, requiring blood levels to ensure efficacy while preventing toxicity, it is also a dangerous drug in the hands of a suicidal patient and has many side effects some patients find intolerable.30 Neither lithium nor valproate are officially indicated by Health Canada or the FDA for bipolar depression. The most recent Canadian bipolar guidelines, published in 2013 by the Canadian Network for Mood and Anxiety Treatments (CANMAT), recommend lithium as first-line therapy for bipolar depression. In the U.S., the Florida Best Practice Guidelines (published in the Journal of Clinical Psychiatry in 2015) suggest a second-line rating for lithium in the treatment of bipolar depression. Until the Canadian guidelines are updated, American recommendations can be a useful tool for helping physicians make informed treatment selection decisions for their patients (within the scope of Health Canada approved agents).
Because patients with bipolar disorder spend most of their symptomatic time in a depressed state, it is imperative that clinicians consider the long-term implications to the whole body when choosing an agent for the treatment of bipolar mania or depression. The adverse effects of medication may be tolerable in the short term, but can add a significant burden to patients if prescribed for long periods of time.
The 2013 CANMAT guidelines recommend lamotrigine as first-line therapy for the acute treatment of bipolar I depression and for maintenance therapy, and as second-line therapy for bipolar II depression. The Florida Best Practice Guidelines rate lamotrigine as a second-line agent based on efficacy. The number needed to treat (NNT) for lamotrigine response, when compared to placebo, is 12 (8-41); the number needed to harm (NNH) is much more favorable.31,32 Aside from the 1/1,000 risk of a serious rash,33 lamotrigine has a tolerability profile superior to AAPs, lithium and valproate. However, the double-digit NNT does not compare favorably to AAPs, lithium or valproate. So despite a NNH that is 3-times higher than the NNT, the favorable benefit/harm ratio is offset by inadequate efficacy. Lamotrigine’s use is also limited for some patients because it is not an effective acute or preventive treatment for mania or hypomania.
In 2011, the Lancet published a systematic review, including 68 randomized controlled trials (16,073 participants), that found that antipsychotic drugs were “strikingly” more effective than mood stabilisers in the management of acute mania.34 Similarly, another meta-analysis of 38 studies involving 10,800 patients demonstrated responses to various antipsychotics were somewhat greater or more rapid than lithium, valproate or carbamazepine in acute mania.35
As a class, AAPs offer several important advantages over conventional antipsychotic agents. Arguably, the reduction in movement disorders has the strongest evidence,36,37 although a reduction of negative symptoms and an improved sense of well-being are also commonly described by patients.38,39 Most AAPs, through their impact on a variety of receptors, also improve depression and anxiety symptoms, as well as conferring mood stabilizing, anti-manic and antipsychotic effects.40 In contrast, many typical antipsychotics, while very useful in the treatment of acute mania, lack good maintenance data and may provoke depression. Because of their breadth of potential symptom amelioration and well-established evidence for short-term and long-term efficacy, AAPs have become first-line choices for bipolar disorder, whether the first presentation is mood elevation or depression. There is also evidence for the value of employing a combination of an AAP and either lithium or valproate, which may improve overall efficacy by 20% compared to either drug alone.41-46
Unfortunately, many AAPs are burdened with tolerability issues to varying degrees—not all are the same.47 The AAPs that have strong empirical evidence for efficacy in acute bipolar depression, including quetiapine, lurasidone and olanzapine (plus fluoxetine), may not be well tolerated by all patients.48,49 Patients with bipolar disorder have an increased risk of metabolic disorders, the cause of which is multifactorial and includes the impact of the underlying disease state, lifestyle factors, and treatment factors.50-54 The metabolic risks associated with olanzapine, and to a somewhat lesser degree with quetiapine, are an added burden for a population that already has an increased risk for cardiometabolic disorders.55 The presence of metabolic syndrome is associated with a more complex bipolar presentation, less favourable response to treatment, and adverse course and outcome. The significant risk of weight gain in bipolar patients is negatively associated with cognitive function56 and highly associated with treatment non-compliance.57 Compared to unipolar depression, bipolar depression is associated with twice the risk of endocrine, cardiovascular, and cerebrovascular morbidity and mortality.58
Fortunately, there have been important advancements regarding metabolic side effect burden with some of the newer AAPs.59 While their classification suggests homogeneity, AAPs have heterogeneous safety and tolerability profiles. The most recently available AAPs, aripiprazole, lurasidone, asenapine and ziprasidone, have more favorable metabolic profiles (Table 1).60 Of the group that has demonstrated efficacy in bipolar depression, lurasidone’s tolerability, when compared to quetiapine and olanzapine, may be associated with greater compliance due to a generally positive tolerability profile.61-63
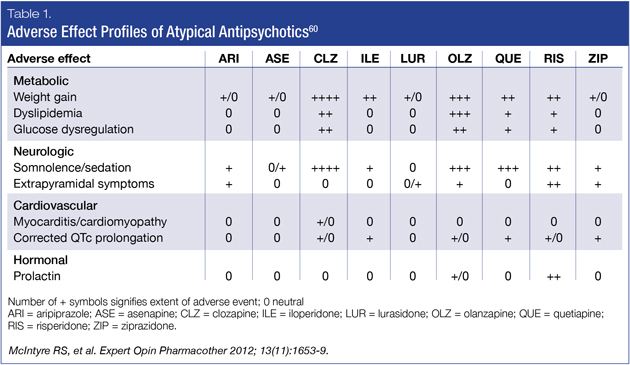
Numbers Need to Treat and Numbers Needed to Harm
Examining NNT compared to NNH amongst AAPs approved to treat bipolar depression can be a helpful approach when selecting one treatment over another. A low NNT is desired, accompanied by a high NNH. A comparative table of approved AAPs and their respective NNTs and NNHs are shown in Table 2.47
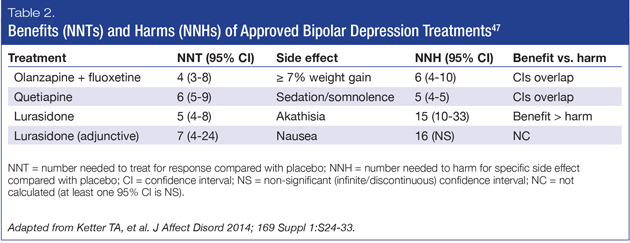
The Florida Best Practice Guidelines64 recommend lurasidone monotherapy or lurasidone prescribed in combination with lithium or valproate as first-line treatment for bipolar I depression. Lurasidone monotherapy, when compared with placebo, had a NNT for response of 5 (4-8) and a NNH for akathisia of 15 (10-33),60 demonstrating that lurasidone monotherapy is efficacious and more likely to yield benefit (response) than harm (akathisia). When compared with placebo, lurasidone prescribed in conjunction with lithium or valproate, had a NNT for response of 7 (4-24) and an NNH for nausea of 16 (95% confidence interval was not significant),61 suggesting this combination is more likely to yield benefit (response) than harm (nausea). Thus, lurasidone, whether prescribed alone or as adjunctive therapy, has a favorable benefit/harm ratio.
Quetiapine, both as monotherapy and in combination with lithium and valproate, is also recommended first-line treatment by the Florida Best Practice Guidelines and as first-line monotherapy by the 2013 CANMAT guidelines,65 for both bipolar I and II depression. Quetiapine has comparable efficacy to lurasidone as monotherapy, with a NNT for response when compared to placebo of 6 (5-9). However, its NNH for sedation and somnolence is 5 (4-5).46,66 While the efficacy of olanzapine with fluoxetine is well established, it is considered second-line treatment in the Florida Best Practice Guidelines and the CANMAT guidelines due to weight and metabolic side effects. When compared to placebo, olanzapine plus fluoxetine has a NNT for response of 4 (3-8) and a NNH of 6 (4-10) for ≥ 7% weight gain.48 Consequently, while both quetiapine and the combination of olanzapine and fluoxetine have single-digit NNTs, when compared to placebo, they also have single-digit NNHs. While this demonstrates clinical efficacy, they are just as likely to cause side effects that some patients might find intolerable.
Summary
While it is essential to rapidly and effectively treat acute mania, long-term mood stability and prophylaxis are critical considerations. Though effectiveness is a core component of treatment choice, so too is tolerability. Negative health issues associated with some agents, particularly related to weight gain and metabolic syndrome, not only impact patient quality of life but also contribute to the burden of disease by compounding the symptoms of depression. For optimal treatment outcomes, both in the short term and the long term, therapy for bipolar disorder should be selected for the individual patient to maximize efficacy and minimize negative consequences to overall physical health. Clinicians support the best short-term and long-term quality of life for their patients with bipolar disorder when they treat the mind and also respect the body.
Development of this article was funded by Sunovion Pharmaceuticals Canada Inc. The author had complete editorial independence in the development of this article and is responsible for its accuracy and completeness. Editorial assistance was provided by STA HealthCare Communications.
References
1. Hirschfeld RM, Lewis L, Vornik LA. Perceptions and impact of bipolar disorder: how far have we really come? Results of the national depressive and manic-depressive association 2000 survey of individuals with bipolar disorder. J Clin Psychiatry 2003; 64(2):161-74.
2. Baldessarini RJ, Tondo L, Baethge CF, et al. Effects of treatment latency on response to maintenance treatment in manic-depressive disorders. Bipolar Disord 2007; 9(4):386-93.
3. Mitchell PB, Goodwin GM, Johnson GF, et al. Diagnostic guidelines for bipolar depression: a probabilistic approach. Bipolar Disord 2008; 10(1 Pt 2):144-52.
4. Schaffer A, Cairney J, Veldhuizen S, et al. A population-based analysis of distinguishers of bipolar disorder from major depressive disorder. J Affect Disord 2010; 125(1-3):103-10.
5. Hirschfeld RM, Cass AR, Holt DC, et al. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Pract 2005; 18(4):233-9.
6. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry 2002; 59(6):530-7.
7. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry 2003; 60(3):261-9.
8. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry 2011; 68(3):241-51.
9. Magalhães PV, Kapczinski F, Nierenberg AA, et al. Illness burden and medical comorbidity in the Systematic Treatment Enhancement Program for Bipolar Disorder. Acta Psychiatr Scand 2012; 125(4):303-8.
10. Benton T, Staab J, Evans DL. Medical co-morbidity in depressive disorders. Ann Clin Psychiatry 2007; 19(4):289-303.
11. McDermut W, Mattia J, Zimmerman M. Comorbidity burden and its impact on psychosocial morbidity in depressed outpatients. J Affect Disord 2001; 65(3):289-95.
12. Rush AJ, Warden D, Wisniewski SR, et al. STAR*D: revising conventional wisdom. CNS Drugs 2009; 23(8):627-47.
13. Hirschfeld RM, Vornik LA. Bipolar disorder—costs and comorbidity. Am J Manag Care 2005; 11(3 Suppl):S85-90.
14. Kessler RC, Berglund P, Demler O, et al., The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). 2003; 289(23):3095-105.
15. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry 2007; 64(5):543-52.
16. Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet 1997; 349(9063):1436-42.
17. Costa Lda S, Alencar AP, Nascimento Neto PJ, et al. Risk factors for suicide in bipolar disorder: a systematic review. J Affect Disord 2015; 170:237-54.
18. Pompili M, Gonda X, Serafini G, et al. Epidemiology of suicide in bipolar disorders: a systematic review of the literature. Bipolar Disord 2013; 15(5):457-90.
19. Bellivier F, Etain B, Malafosse A, et al. Age at onset in bipolar I affective disorder in the USA and Europe. World J Biol Psychiatry 2014; 15(5):369-76.
20. Perlis RH, Miyahara S, Marangell LB, et al. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD). Biol Psychiatry 2004; 55(9):875-81.
21. Joslyn C, Hawes DJ, Hunt C, et al. Is age of onset associated with severity, prognosis, and clinical features in bipolar disorder? A meta-analytic review. Bipolar Disord 2016; 18(5):389-403.
22. Valentí M, Pacchiarotti I, Undurraga J, et al. Risk factors for rapid cycling in bipolar disorder. Bipolar Disord 2015; 17(5):549-59.
23. Vieta E, Grunze H, Azorin JM, et al. Phenomenology of manic episodes according to the presence or absence of depressive features as defined in DSM-5: Results from the IMPACT self-reported online survey. J Affect Disord 2014; 156:206-13.
24. Yatham LN, Kennedy SH, Schaffer A, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2009. Bipolar Disord 2009; 11(3):225-55.
25. Kleimann A, Schrader V, Stübner S, et al. Psychopharmacological treatment of 1650 in-patients with acute mania-data from the AMSP study. J Affect Disord 2016; 191:164-71.
26. Malhi GS, Adams DA, Berk M. Medicating mood with maintenance in mind: bipolar depression pharmacotherapy. Bipolar Disord 2009; 11 Suppl 2:55-76.
27. Frye MA, Ketter TA, Altshuler LL, et al. Clozapine in bipolar disorder: treatment implications for other atypical antipsychotics. J Affect Disord 1998; 48(2-3):91-104.
28. Bowden CL, Brugger AM, Swann AC, et al. Efficacy of divalproex vs lithium and placebo in the treatment of mania. The Depakote Mania Study Group. JAMA. 1994; 271(12):918-24.
29. Geddes JR, Goodwin GM, Rendell J, et al. Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open-label trial. Lancet 2010; 375(9712):385-95.
30. Miura T, Noma H, Furukawa TA, et al. Comparative efficacy and tolerability of pharmacological treatments in the maintenance treatment of bipolar disorder: a systematic review and network meta-analysis. Lancet Psychiatry 2014; 1(5):351-9.
31. Calabrese JR, Huffman RF, White RL, et al. Lamotrigine in the acute treatment of bipolar depression: results of five double-blind, placebo-controlled clinical trials. Bipolar Disord 2008; 10(2):323-33.
32. Geddes JR, Calabrese JR, Goodwin GM. Lamotrigine for treatment of bipolar depression: independent meta-analysis and meta-regression of individual patient data from five randomised trials. Br J Psychiatry 2009; 194(1):4-9.
33. GlaxoSmithKline Inc. Lamictal® product monograph. Date of revision: October 5, 2016.
34. Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet 2011; 378(9799):1306-15.
35. Yildiz A, Vieta E, Leucht S, et al. Efficacy of antimanic treatments: meta-analysis of randomized, controlled trials. Neuropsychopharmacology 2011; 36(2):375-89.
36. Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry 2004; 161(3):414-25.
37. O’Brien A. Comparing the risk of tardive dyskinesia in older adults with first-generation and second-generation antipsychotics: a systematic review and meta-analysis. Int J Geriatr Psychiatry 2016; 31(7):683-93.
38. Mohr P. Quality of life in the long-term treatment and the role of second-generation antipsychotics. Neuro Endocrinol Lett 2007; 28 Suppl 1:117-33.
39. Orsolini L, Tomasetti C, Valchera A, et al. An update of safety of clinically used atypical antipsychotics. Expert Opin Drug Saf 2016; 15(10):1329-47.
40. Seeman P. Atypical antipsychotics: mechanism of action. Can J Psychiatry 2002; 47(1):27-38.
41. Sachs G, Chengappa KN, Suppes T, et al. Quetiapine with lithium or divalproex for the treatment of bipolar mania: a randomized, double-blind, placebo-controlled study. Bipolar Disord 2004; 6(3):213-23.
42. Sachs GS, Grossman F, Ghaemi SN, et al. Combination of a mood stabilizer with risperidone or haloperidol for treatment of acute mania: a double-blind, placebo-controlled comparison of efficacy and safety. Am J Psychiatry 2002; 159(7):1146-54.
43. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry 2002; 59(1):62-9.
44. Vieta E, T’Joen C, McQuade RD, et al. Efficacy of adjunctive aripiprazole to either valproate or lithium in bipolar mania patients partially nonresponsive to valproate/lithium monotherapy: a placebo-controlled study. Am J Psychiatry 2008; 165(10):1316-25.
45. Yatham LN, Grossman F, Augustyns I, et al. Mood stabilisers plus risperidone or placebo in the treatment of acute mania. International, double-blind, randomised controlled trial. Br J Psychiatry 2003; 182:141-7.
46. Data on file. Sunovion Pharmaceuticals Inc.
47. Ketter TA, Miller S, Dell’Osso B, et al. Balancing benefits and harms of treatments for acute bipolar depression. J Affect Disord 2014; 169 Suppl 1:S24-33.
48. Calabrese JR, Keck PE Jr, Macfadden W, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry 2005; 162(7):1351-60.
49. Tohen M, Vieta E, Calabrese J, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 2003; 60(11)1079-88.
50. McElroy SL, Keck PE Jr. Metabolic syndrome in bipolar disorder: a review with a focus on bipolar depression. J Clin Psychiatry 2014; 75(1):46-61.
51. Birkenaes AB, Opjordsmoen S, Brunborg C, et al. The level of cardiovascular risk factors in bipolar disorder equals that of schizophrenia: a comparative study. J Clin Psychiatry 2007; 68(6):917-23.
52. Taylor V, McKinnon MC, Macdonald K, et al. Adults with mood disorders have an increased risk profile for cardiovascular disease within the first 2 years of treatment. Can J Psychiatry 2010; 55(6):362-8.
53. Hasnain M, Vieweg WV. Weight considerations in psychotropic drug prescribing and switching. Postgrad Med 2013; 125(5):117-29.
54. Hasnain M, Vieweg WV, Hollett B. Weight gain and glucose dysregulationwith second-generation antipsychotics and antidepressants: a review for primary care physicians. Postgrad Med 2012; 124(4):154–167.
55. McIntyre RS, Danilewitz M, Liauw SS, et al. Bipolar disorder and metabolic syndrome: an international perspective. J Affect Disord 2010; 126(3):366-87.
56. Yim CY, Soczynska JK, Kennedy SH, et al. The effect of overweight/obesity on cognitive function in euthymic individuals with bipolar disorder. Eur Psychiatry 2012; 27(3):223-8.
57. Lett TA, Wallace TJ, Chowdhury NI, et al. Pharmacogenetics of antipsychotic-induced weight gain: review and clinical implications. Mol Psychiatry 2012; 17(3):242-66.
58. Osby U, Brandt L, Correia N, et al. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry 2001; 58(9):844-50.
59. Cha DS, McIntyre RS. Treatment-emergent adverse events associated with atypical antipsychotics. Expert Opin Pharmacother 2012; 13(11):1587-98.
60. McIntyre RS, Cha DS, Alsuwaidan M, et al. A review of published evidence reporting on the efficacy and pharmacology of lurasidone. Expert Opin Pharmacother 2012; 13(11):1653-9.
61. Ketter TA, Sarma K, Silva R, et al. Lurasidone in the long-term treatment of patients with bipolar disorder: a 24-week open-label extension study. Depress Anxiety 2016; 33(5):424-34.
62. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry 2014; 171(2):160-8.
63. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry 2014; 171(2):169-77.
64. 2015 Florida Best Practice Psychotherapeutic Medication Guidelines for Adults. The University of South Florida, Florida Medicaid Drug Therapy Management Program 2015. Accessed online at: http://www.medicaidmentalhealth.org/ViewGuideline.cfm?GuidelineID=97.
65. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord 2013; 15(1):1-44.
66. Thase ME, Macfadden W, Weisler RH, et al. Efficacy of quetiapine monotherapy in bipolar I and II depression: a double-blind, placebocontrolled study (The BOLDER II Study). J Clin Psychopharmacol 2006; 26(6):600-9.